1.5 Lewis structures of ions
So far, you have only written Lewis structures for neutral molecules, but they can also be drawn for ions, such as the hydroxide ion, HO− and the ammonium ion, NH4+.
However, to take the charges into account you need to begin by adding or subtracting electrons from particular atoms.
One way of giving chemically plausible structures is to add or subtract electrons from the atom of highest valency.
So as the hydroxide ion carries a single negative charge, you need to add one electron to the shell structure of the oxygen atom.
This means the Lewis structure for OH- is written as shown in structure 4.18:
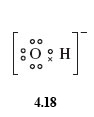
Note again, each atom possesses a noble gas structure.
What shell structure in the ammonium ion is adjusted in order to write a Lewis structure?
Nitrogen has a higher valency than hydrogen, so the single positive charge on the ammonium ion requires the removal of one electron from the nitrogen atom shell structure.
Sketch a Lewis structure for the ammonium ion.
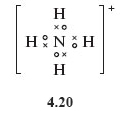
Again each atom possesses a noble gas structure.
In fact it is remarkable how many molecules and ions can be represented by Lewis structures in which each atom has a noble gas shell structure.
Nevertheless, many exceptions do exist and you will look at a few examples in the next section.
