2.3 Is the rate of reaction very slow?
If the equilibrium position is very favourable, then the reason why Reaction (1) fails to occur at 525 °C must be that its rate is very slow.
Usually, a reasonable response would be to increase the temperature further, but the structure and economy of the car provides little scope to do this. The alternative is to use a catalyst, which leaves the equilibrium constant unchanged, while speeding the reaction up.
Internal energy is the sum of the kinetic and potential energies of the component particles of the system being considered. If you look at the changes that take place in the internal energy as reactants change progressively into products. Figure 2 shows a simplified version. The internal energies of the reactants (2NO + 2CO) and products (N2 + 2CO2) are marked by two 'platforms'. The platform for the products lies lower than that for the reactants. This shows that the internal energy change during the reaction is negative.
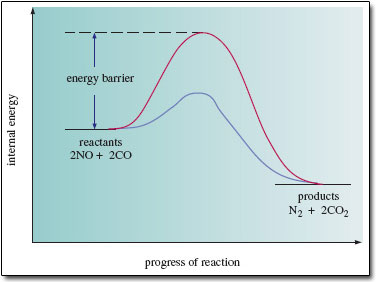
Between the reactants and products, the internal energy does not decrease gradually as the reaction progresses; instead, it rises initially, reaches a maximum, and then declines. The upper curve shows the situation in the absence of a catalyst. The internal energy of the reacting molecules must first increase by an amount marked 'energy barrier' in Figure 2.
How might the reactants, NO and CO, acquire this additional energy?
One possible source is the kinetic energy of other molecules. Lucky collisions may provide some NO and CO molecules with unusually high energies. If these high-energy molecules then chance to collide with each other, they might be able to surmount the energy barrier and react together.
This also explains why an increase in temperature increases the rate of a reaction: a temperature rise increases the speed of the molecules, and the required increase in internal energy following collisions then becomes more probable.
But as we have seen, in this case the rate is not great even at 525 °C, the energy barrier must be very high.
The main reason is a property of nitric oxide, looking at Reaction 1, can you suggest what this might be?
In Reaction 1, the nitrogen and oxygen atoms in NO must be separated at some point, this involves breaking the bond between the two atoms, a reasonable thought is that this bond is strong.
Indeed the bond that holds the nitrogen and oxygen atoms together is very strong – it is a triple bond. A large input of energy is therefore needed to bring the separation about, so the energy barrier is high and the reaction is slow.
The solution to the high energy barrier for Reaction 1 is to involve a third party – a catalyst. What happens at the molecular level, and how the catalyst is considered, is covered in the next section.
