1.4 Alcohol counterfeiting and contamination
Yet another consideration in brewing beer is product quality and ensuring that alcohol has not been contaminated with any other substances.
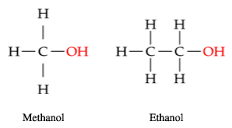
Have a look at the following two compounds illustrated in Figure 3. Thinking back to Week 1, can you list some similarities between them? Can you identify one or both of these compounds?
The two compounds:
- are both examples of alcohols as they possess the −OH functional group
- both consist of C (carbon), H (hydrogen) and O (oxygen) atoms
- both consist of single bonds.
From Week 1, you may recall that the compound on the left is methanol and has the formula CH3OH. The compound on the right is ethanol and has the formula C2H5OH.
While these compounds may look similar, they do in fact have very different properties. Ethanol is the key component of alcoholic beverages, and you looked at the effects of ethanol on human health in Weeks 5 and 7.
Methanol, in comparison, is the simplest form of alcohol – it is closely related to ethanol but much more toxic. Methanol is a very undesirable compound in alcoholic beverages as it is very harmful to the human body. Methanol is converted in the body into formic acid, the same toxin that is found in the venom of ants. It is this accumulation of formic acid in the blood that causes the symptoms of methanol poisoning – kidney failure, heart and circulation problems, liver damage, and visual disturbances (blurred and tunnel vision, changes in colour perception, and temporary or permanent blindness).
Rather worryingly, there have been cases of methanol poisoning which have arisen from consumption of alcohol contaminated with methanol. As little as 30 ml could be fatal. Methanol is used in antifreeze, as a solvent, as fuel, and as a denaturant (an additive that makes otherwise potable alcohol unfit for human consumption), for example in methylated spirits. All of these sources of methanol have been – and are still being – used by spirits counterfeiters since they are cheap and easily accessible.
So it is obviously important to be able to detect the presence of methanol in alcoholic beverages. One analytical chemistry technique that can detect the presence of methanol (and other contaminants) in alcohol is mass spectrometry.