Centrifugation
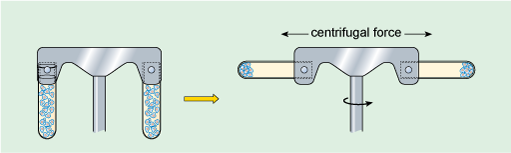
Large particles suspended in liquid will eventually settle out under the influence of the Earth's gravitational field (the strength of this force is denoted g). Small particles, however, will usually separate extremely slowly (or not at all) unless subjected to a much greater force than gravity. This can be achieved by centrifugation, in which the suspension of particles is spun round an axis at very high rotational speeds (Figure 4), creating what is commonly referred to as a centrifugal force, which causes particles to migrate away from the axis. The rate at which particles settle out (or sediment) depends on their size, shape and density (density is the mass of a given volume of a substance, i.e. density = mass/volume), the density of the liquid, and the rotational speed of centrifugation. Centrifugation at different speeds allows the separation of particles into 'fractions', according to how readily they sediment (Box 1). For example, at low centrifugation speeds, large cells can be separated from small cells. In the 1940s and '50s, advances in the technology of centrifugation allowed samples to be spun at very high speeds indeed, a refinement known as ultracentrifugation. For example, a centrifugation speed of 30 000 revolutions per minute (rpm) can produce a centrifugal force of 100 000 times Earth's gravitational force (100 000 × g). Ultracentrifugation using the appropriate types of sample preparation and solutions allows the separation of very small particles such as organelles and even macromolecules.
Box 1 Cell fractionation
When a suspension of cells is placed in an appropriate physiological solution (an artificially prepared solution, which has pH and salt concentrations that mimic the internal environment of the cell) and disrupted, for example by detergents or ultrasound, or by homogenisation, many of the organelles such as nuclei, mitochondria and Golgi apparatus (Figure 1a and b) remain intact. The homogenisation apparatus consists of a tube with a tightly fitted pestle that is rotated to shear the cells (Figure 5a). The cell membrane and the endoplasmic reticulum are disrupted, but their membranes can re-seal to form vesicle-like structures called microsomes. If conditions are right, the original chemical properties of the organelles and membranes are retained during the preparation.
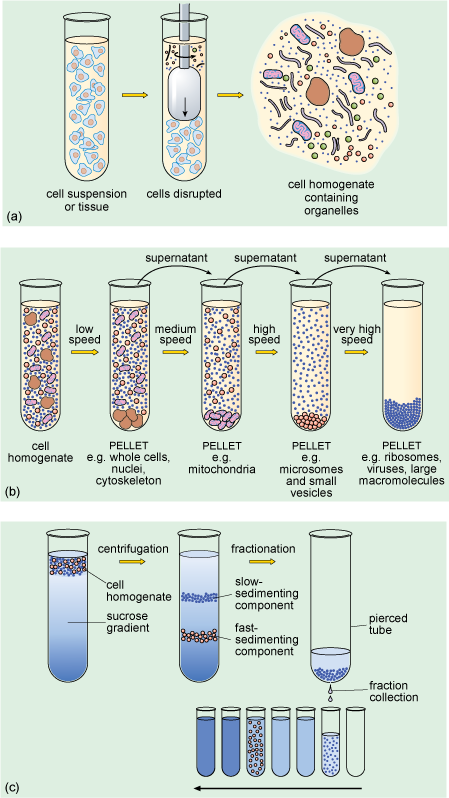
The subcellular components can then be recovered by centrifugation. The cell homogenate is dispensed into centrifuge tubes which are placed into a rotating holder (known as a rotor) that fits into the centrifuge. As the rotor turns, particles suspended in the homogenate migrate towards the bottom of the tube. Particles collected at the bottom of the tube form a 'pellet'. Since larger components travel fastest, they can be separated from smaller particles by differential sedimentation (Figure 5b), in which centrifugation at progressively higher speeds is used to separate particles in the homogenate according to their size and density. After an initial centrifugation, the pellet, containing the largest components, is separated from the remaining suspension (known as the supernatant) which contains the smaller components. The pellet can be re-suspended and its components studied, while the supernatant can be transferred to another centrifuge tube and spun at a higher speed to recover smaller components into another pellet. A series of such steps can be performed to separate the different components of a cell homogenate.
A problem with differential sedimentation is that separation is not complete; pellets actually contain something of a mixture of cellular components. A more sensitive and widely used method is density gradient centrifugation in which the cell lysate is laid on top of a density gradient. This is prepared in a centrifuge tube by layering sucrose solutions of varying densities, the solutions becoming denser towards the bottom of the tube. Movement through the density gradient optimises the separation of particles of different density and size into different levels or bands in the gradient, so that they can be removed as separate 'fractions' (Figure 5c), either by using a fine pipette or by piercing the bottom of the tube and collecting the fractions as the liquid drips out.
Once separation is achieved, the biochemical properties of the different fractions can be analysed. Fractionation methods have been of great importance in cell biology because they enabled the functions of the different cell organelles to be elucidated. Indeed this method provided the first evidence for the existence of some organelles, such as lysosomes and peroxisomes (small membrane-bound organelles that contain different types of degradative enzymes, described later in this course).