3.5.1 The mica group
Mica is a general name given to a range of sheet silicate minerals that are commonly found in igneous, metamorphic and sedimentary rocks. In igneous rocks, they crystallise from hydrous magmas with medium to high silica contents; in metamorphic rocks the parallel alignment of mica crystals defines the foliation found in slates and schists.
Micas have sandwich structures, weakly bonded by interlayer ions. Each sandwich contains a tetrahedral sheet on each side of an octahedral sheet (Figure 48d). Commonly one in four of the tetrahedra contains Al instead of Si (although the number of oxygen atoms remains unchanged), with the result that sheets have an excess negative charge. This is balanced by the presence of interlayer cations, such as K+, between the sandwiches (Figure 49a).

The bonding inside a sandwich is very strong, but between sandwiches it is very weak (due to the interlayer ions), permitting one sandwich to slide past another. Thus, mica has one perfect cleavage, parallel to the layers, so it is easy to split a mica crystal into very thin flakes (see Digital Kit [Tip: hold Ctrl and click a link to open it in a new tab. (Hide tip)] ).
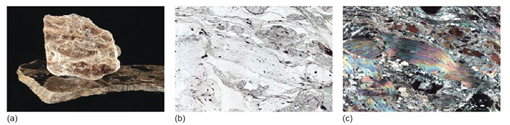
You have seen that there are two options for making the octahedral layers: either an Al(OH)3 dioctahedral layer (Figure 48c), or a Mg(OH)2 trioctahedral layer (Figure 48b). These give rise to two important mica minerals: muscovite ('white' mica; Figure 51a), which contains dioctahedral layers, and biotite ('dark' mica; Figure 50a), which contains trioctahedral layers in which Fe2+ commonly substitutes for Mg2+.

-
Why should biotite commonly be dark and muscovite be white?
-
Silicate minerals containing large amounts of Fe tend to have dark colours, and this is also true of biotite, which is often Fe rich. Muscovite contains little if any Fe, and so is usually pale coloured.
The structures of muscovite and biotite are given, in schematic form, in Figure 49a.
Micas are striking minerals under the microscope; biotites are often strongly coloured, and muscovites are colourless in plane-polarised light, but both have a perfect cleavage and vivid second- to third-order interference colours. The difference in colour between biotite, with strong pleochroism, and muscovite is diagnostic in thin section (Figures 50b and c; 51b and c). Note that basal sections show neither cleavage nor pleochroism.
