3.6.1 Silica minerals
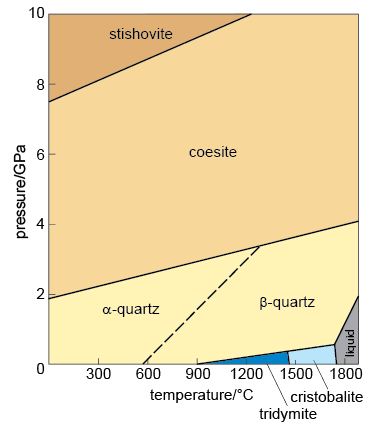
The silica minerals have fully polymerised structures and are essentially pure silica. There are several minerals with the chemical formula SiO2, although most of them are usually referred to as quartz, as it is difficult to recognise the differences between them in both hand specimen and thin section. The SiO2 phase diagram (Figure 52) illustrates that at least five forms of SiO2 can be found in the Earth's crust and a further high-pressure form called stishovite is stable at pressures greater than 7.5 GPa. At progressively higher pressures, the Si and O atoms are packed closer together so that coesite is denser than α-quartz and stishovite is denser than coesite. Silica minerals are very common in sedimentary, igneous and metamorphic rocks because they are chemically and mechanically stable under a wide range of conditions. Figure 52 can be used to predict which forms of SiO2 are found in different rocks: α-quartz is found in sedimentary rocks whereas β-quartz is found in high-temperature/low-pressure metamorphic rocks. Coesite is a characteristic mineral of some high-pressure metamorphic rocks whereas stishovite has only ever been discovered in nature in meteorite impact sites where very high pressures and temperatures were developed. In igneous rocks, tridymite and cristobalite are restricted to lavas that crystallised rapidly from high-temperature silica-rich magmas.
SAQ 7
What pressure would be required to convert silica into stishovite at 300 °C?
The strong, three-dimensional bonding in quartz, which is dominantly covalent on account of there being no metal cations present, means that there are no definite planes of weakness and, therefore, no cleavage. So, when broken, quartz shatters to form glassy fragments with a curved, conchoidal fracture. The strong bonding also makes quartz very hard and extremely resistant to chemical attack. Consequently, quartz grains can survive transport by wind or water over vast distances, eventually being deposited as sand grains.
Although quartz is very pure SiO2, it can sometimes contain small amounts of impurities such as aluminium, iron, lithium and titanium. The effect of these impurities is to produce quite dramatic colours in what would otherwise be an entirely colourless mineral (Figures 7 and 53a).

Under the microscope, quartz lacks cleavage and colour and has low first-order, grey-white interference colours (Figure 53b and c). Being chemically stable, quartz crystals look clean compared with feldspars, which are almost always turbid or cloudy.
Activity 3.5 Quartz in hand specimen and thin section
This activity will help you to recognise quartz in hand specimen and thin section. For this activity you will need to look at quartz and granite in the Digital Kit [Tip: hold Ctrl and click a link to open it in a new tab. (Hide tip)] and granite in the Virtual Microscope.
Although quartz crystals can grow to form large, well-developed single crystals (such as the well-formed crystals shown in the Digital Kit), it is more common to find quartz crystals as irregular-shaped grains in rocks, often filling the gaps between other crystals.
- Examine the granite sample, and particularly the close-up image in the Digital Kit. This contains dark mica crystals (biotite) plus a white mineral. It also contains grey/glassy grains, which are quartz crystals. Note that they have no cleavage.
- Look at the granite in thin section using the Virtual Microscope (found under the ‘Igneous rocks’ category) in plane-polarised light. The quartz crystals have very low relief, and are almost invisible. When viewed between crossed polars they show low-order, grey interference colours and, unlike the other low-relief mineral in the thin section, they do not feature any regular kind of patterning indicative of twinning (see Activities 3.6 and 3.7).
- When viewed between crossed polars, some of the quartz crystals, such as the one featured in View 4, do not snap in and out of extinction as uniformly as other minerals in the thin section. Instead, they show undulose or 'wavy' extinction, whereby different parts of a crystal are in extinction at slightly different positions from other parts (see also coordinates 2400,800). This effect is because the structures of different regions of the crystal are slightly out of alignment with each other, due to the quartz crystal lattices having been stressed. This is especially common in metamorphic rocks, but can occur in other rocks that either contain relict crystals (reflecting an earlier history), or have been deformed after formation.
