3.7 Non-silicate minerals
3.7.1 Carbonates
The most common carbonate mineral is calcite (CaCO3) (Figure 61a). Calcite is one polymorph of CaCO3. Another polymorph is aragonite, which has a different crystal structure - it is orthorhombic rather than trigonal. Aragonite is less stable than calcite under ambient conditions. Many marine organisms initially build their skeletons of aragonite but, when they die, their shells drop to the sea floor and gradually the aragonite transforms into calcite.
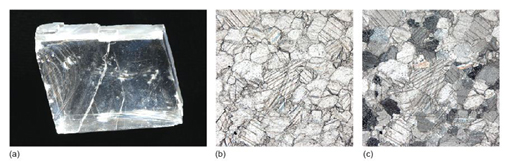
Calcite is the major constituent of limestone rocks. Limestones are important industrial minerals; they are used not only as aggregates in the construction industry and in powdered form as filler for plastics, paints and rubber, but also as a major constituent of cement.
In thin section, calcite is distinctive: it is a highly anisotropic mineral, with high-order interference colours (Figure 61c). In hand specimen it is quite a soft mineral (hardness 3 on Mohs' scale, Table 2) and has three excellent cleavages (Figure 61a). Dilute (5-10%) hydrochloric acid (HCl) provides a good test for calcium carbonate in hand specimen. A few drops, carefully applied, will fizz, giving off carbon dioxide:
Dolomite (CaMg(CO3)2) is another carbonate mineral that is found in many limestones. Dolomite rocks form when there is an excess of Mg ions available, either during their formation or during later burial.
