7.1 Some aspects of the science of plastics
Let’s spend a few moments looking at some of the science that underlies plastics and their production.
Plastics are comprised of so-called polymer molecules, where a long molecular chain is formed from a repeating molecular unit. (This name derives from the Greek: poly + meros = ‘many’ + ‘parts’). Furthermore, the term ‘polymer’ explains the use of the term ‘poly’ in the chemical names of plastics that you met in the previous section.
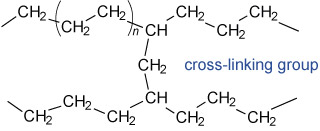
As an example, let us consider the relatively simple structure of polyethene, which is, at a basic level, the molecule (CH2CH2)n, where n is a large number resulting in a molecule with a long chain (see Figure 3).
The value of n, and how the individual chains are connected to each other (which is known as cross-linking; see the CH–CH2–CH arrangement next to the blue label in Figure 3), largely determines the properties observed for the plastic. These properties include the plastic’s density and melting temperature, which can be varied by altering the chemical production process.
This makes plastics like polythene highly versatile, and some everyday variations include:
- low density polyethene (LDPE), which is used in food packaging trays, wire insulation
- medium density polyethene (MDPE), which is used in carrier bags and shrink film
- high density polyethene (HDPE), which is used in milk bottles, soft drink bottle caps, pipes and some surgical implants.
Other chemicals may be added to the plastic to change the colour, act as antioxidants, improve how it wears or increase its plasticity or fluidity, where the latter chemicals are called plasticisers. However, additives in plastics sometimes cause health and environmental concerns if they leach out of the material, and this is an active area of research.
Question 2 Plastic degradation
From your own observations, how easily do plastics degrade in the environment?
Polyethene is rather chemically inert; this is a property that may be either beneficial or problematic during the lifetime of a product made from it. Video 4, dating from September 2015, considers some aspects of polyethene in the environment, and one way that scientific research is progressing for plastics.

Transcript: Video 4 New biodegradable materials could replace plastic bags.
Question 3 Chemical inertness
Considering the examples of everyday use given above, and the other information you've read so far, can you suggest when the chemical inertness of polyethene might be useful, and when it might be a problem?
Answer
In food packaging, chemical inertness is a benefit as it means there will be no unwanted reactions that alter the taste of the products. Similarly, when used as a medical implant, there are generally no unwanted reactions to the implant in the patient’s body.
However, at the end of a product’s lifetime, this inertness may become a real problem, depending on how it is treated by society. If it is simply discarded, its lack of reactivity means that it will exist in the environment for a significant time, either as litter on land or pollution in the ocean (see Figure 5).
Video 4 suggested there is estimated to be 5 trillion pieces of plastic, or about 270 000 tonnes, in the oceans. It is noteworthy that this figure only refers to the plastic floating in the ocean (Eriksen et al., 2014). It is also noteworthy that other studies have estimated different quantities of plastic in the oceans. For example, Jambeck et al. (2015), proposed that in 2010 around 4.8 to 12.7 million tonnes entered the oceans. The range represented by the estimates from these two papers illustrates the extent to which we need to further develop our knowledge of exactly what impact plastics are having on our environment. Additionally, we need to develop better waste management for plastic wastes and more sustainable alternatives.
If a waste plastic is to be recycled, then the ease with which this can be performed depends on how many other types of plastic and other materials are present in the complete waste sample. The disposal of waste plastics, as it links to plastic pollution, is currently an important scientific challenge for society, particularly in terms of the efficiency of any proposed recycling processes (Carné Sánchez and Collinson, 2011).
The environmental and human health risks posed by discarded plastics are subject to much debate and research. Undertaking further work in this area could produce a more thorough risk–benefit analysis of the use of plastics in society, but the viewpoint and/or the scope of analysis would have to be carefully defined.
The scope might be narrow when considering the use of a particular plastic item in an application, such as polyester polyethylene terephthalate (PET) in single-use water bottles. Alternatively, it could be much wider, considering the science behind the formation, use and disposal of a particular plastic item. (For example: the use of polyester fibres (including PET) comprising a mix of both new and recycled materials; their application in textiles; and their collection, and the pros and cons of sorting and recycling such materials versus their disposal to landfill or for energy recovery.)
Strong supporting evidence needs to be provided, in the form of references to critically reviewed research and published data, for any such studies. This is often necessary when scientists produce reports for stakeholders, such as the public or government.
Question 4 Plastics and oil
Currently, most plastics are synthesised from oil, so manufacturing plastics might be expected to increase our use of oil. Can you think of a circumstance where this would not be the case?
Answer
If the plastic is used to produce a lightweight container, then the associated use of diesel fuel to transport the containers will be lower overall than it would be if the containers were heavier (for example, transporting soft-drink plastic bottles compared to glass bottles).
Alternatively, if the plastic was derived from a natural source (i.e. it was a bioplastic) then it would be more sustainable than if it had been made from conventional, hydrocarbon polymers.
Shortly you will examine an example of an online article and some discussion of research literature on plastics. An important aspect of reading new materials to aid your understanding is to develop a glossary of new terms. You will now consider briefly how you might go about preparing such a glossary.