4.2 The functional group approach
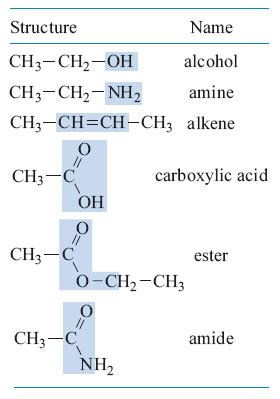
It is the classification of functional groups that simplifies the study of organic chemistry (the chemistry of compounds that contain carbon). With many millions of known organic compounds, and more being added by the day, it would be hopeless if their properties could not be systematised in some way. It turns out that a given functional group usually has the same chemical properties whatever carbon chain it is bonded to, so once the general properties of each functional group are known, all that is left to deal with are the exceptions. It is nearly always the functional groups that undergo change when an organic chemical takes part in a reaction. Fortunately there are relatively few functional groups, some of which are shown in Table 1. For convenience, and to keep the valencies correct, these are shown with a hydrocarbon chain attached. It is the shaded bit of each molecule that is the functional group.
Note that functional groups are parts of molecules but they do not exist in isolation. Molecules of compounds like CH3CH2OH (Table 1) contain the alcohol functional group, —OH, but they also include a hydrocarbon chain, CH3— CH2—. The compound is in the family of compounds called alcohols, all of which have an —OH group attached to a hydrocarbon chain that is often depicted simply by the symbol R.