7.2 How enzymes work
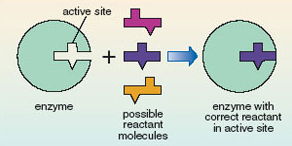
Enzyme molecules have an ‘active site’ that is a specific shape for a given enzyme. It is here that reactant molecules are converted into products. The active site binds to and holds the reactant molecule in exactly the right position for the reaction to take place. Effectively it fits around the molecule rather like a glove fits around a hand. This very precise three-dimensional structure can only be achieved by enzymes being large complex molecules.
Because the enzyme fits the reactant like a glove, the active site will accommodate only a very small range of different molecules. The enzyme is said to be highly specific in its action. The relationship between enzyme and reactant has been likened to a lock and key (Figure 12); the enzyme is like a lock where only one type of key, the correct reactant, will fit the active site. Thus only a very specifically shaped molecule will interact with the enzyme and undergo a reaction. In Figure 12, although the three possible reactant molecules all have very similar shapes, only one can fit into the active site.
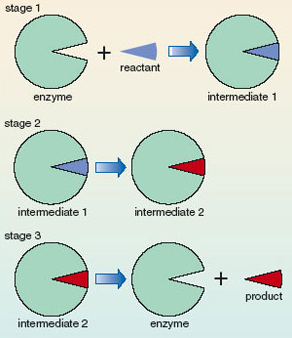
There are usually several steps in a chemical reaction – the reactants do not just disappear and turn into products without passing through a number of intermediate stages. This sequence of intermediate reactions is called the reaction mechanism. Catalysis occurs by the enzyme being intimately involved in the mechanism. Figure 13 shows a schematic representation of the stages that are involved. Enzymes provide an alternative, faster, reaction mechanism. In early stages of the mechanism the enzyme reacts with the reactant to give intermediates; however, the enzyme is regenerated in a later stage. Thus, the catalyst, an enzyme, is not consumed; it is continually recycled so one molecule of enzyme can catalyse the conversion of many reactant molecules into product molecules. Only a small amount of the catalyst is required. Since the catalyst is not consumed, it does not end up as part of the product and the overall reaction product is the same irrespective of whether it is catalysed or not; the catalyst merely speeds up the reaction.
The way that enzymes work is an area of great interest to chemists, because if we know how enzymes control the conversion of reactants into products then it gives the chemist the opportunity to help out when things go wrong.