4.5 Vaccines of the future
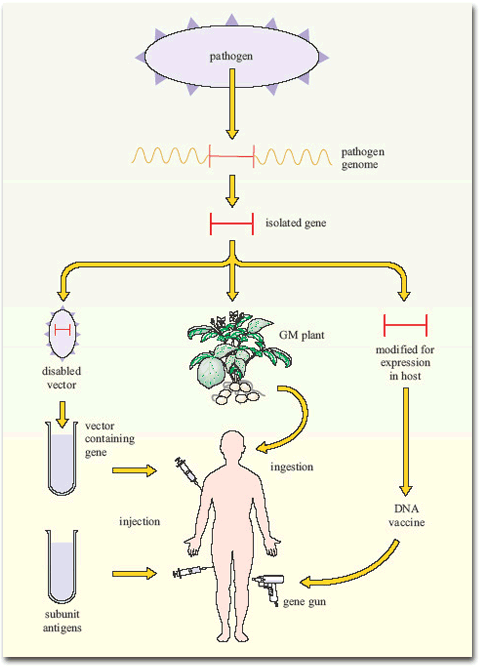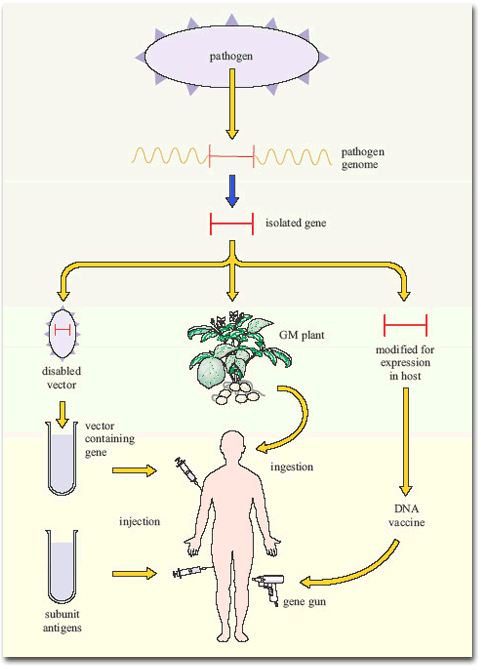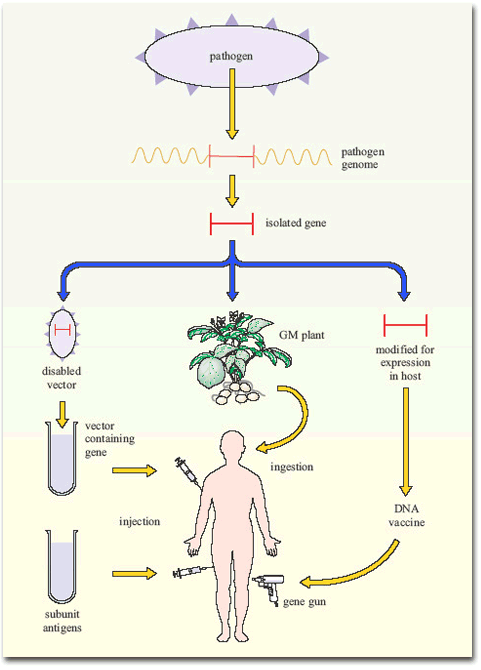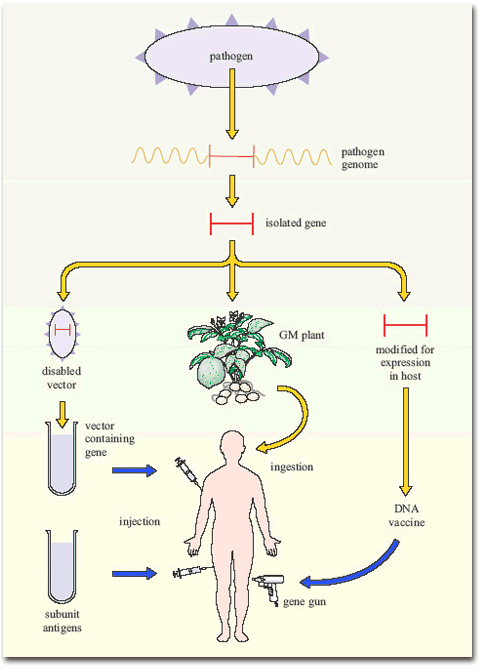
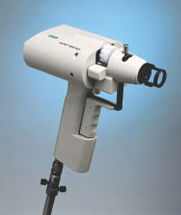
Other applications of modern molecular biology in vaccine design are also coming into play, including DNA vaccines. The use of ‘naked’ (cell-free) DNA encoding critical antigens, directly injected into muscle, seems at first an improbable strategy, since the DNA does not have the necessary cellular machinery for its expression. Nevertheless, if the DNA has a suitable promoter, sufficient can be taken up by cells of the body to allow transcription and production of enough antigen to induce an immune response. The DNA construct is coated onto gold particles which are shot into the tissue using a gas-pressurised ‘gene gun’ (Figure 5). DNA vaccines have been tested in experimental animals with some success, inducing both antibodies and T-cell mediated immunity; trials of DNA constructs from HIV-1 and hepatitis B virus genomes have begun in human volunteers.
Another technique has been to produce genetically-engineered constructs in which the gene encoding a pathogen-specific critical antigen is transported into the vaccine recipient by a harmless virus or bacterium. These vector vaccines immunise the host against the critical antigen, which is expressed by the ‘gene vector’ (Figure 6).
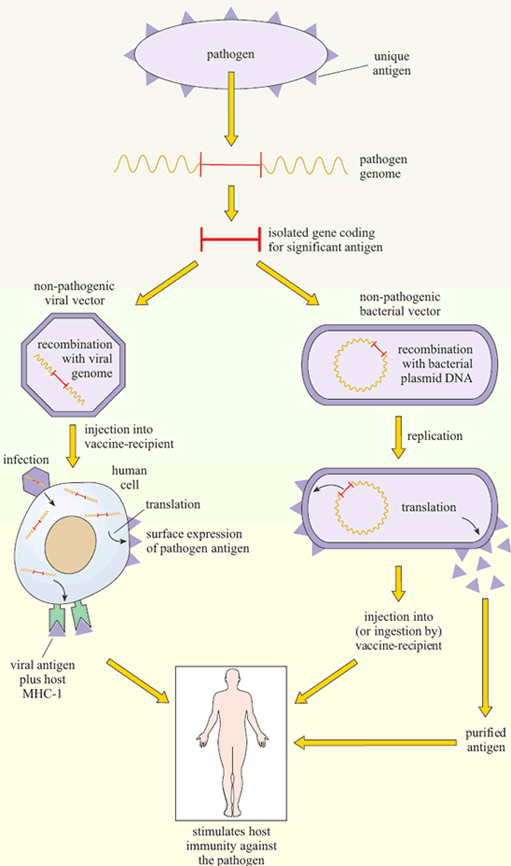
Vaccinia is a popular choice as a gene vector, because it has a long history of relatively safe use in immunisation against smallpox and it can accommodate large amounts of DNA. Moreover, as the use of vaccinia for protection against smallpox has declined since the 1980s, the level of immunity to vaccinia in the population has fallen.
Activity 13
Why is this important in designing a genetically-engineered vaccine assembled in vaccinia viruses?
Answer
If the vaccine recipients are already immune to vaccinia, they would eliminate a vaccinia construct before it could induce immunity to the antigen it was carrying.
Several vector vaccines have been developed to combat HIV infection, so far with limited results. An early version used canarypox virus carrying genes for HIV surface proteins (gp120 and gp41) and encoding some internal molecules including the viral polymerase. A strain of Venezuelan equine encephalitis virus (VEE) has also been used as a vector for gp120. VEE has the advantages of being able to infect human cells and express the HIV glycoprotein, and although it can replicate in human cells, it cannot produce infectious virions. Malaria is another priority target for development of vector vaccines using DNA constructs from Plasmodium carried in vaccinia or fowlpox viruses.
Activity 14
The examples given above all use gene vectors derived from non-human animal viruses: cowpox, canarypox, fowlpox and equine encephalitis. Why are these selected rather than a harmless virus that normally infects humans?
Answer
Firstly, there is the consideration of safety. If a viral vector does not normally infect humans, then it is less likely to revert to a pathogenic type or exchange genetic information with a wild-type human virus. Secondly, recipients will be less likely to have prior immunity to a non-human virus, which would interfere with the development of immunity to the critical antigen in the vaccine.
Bacteria are also under investigation as gene vectors (Figure 6), for example, in the oral typhoid vaccine, Ty21a. There are certain advantages in this approach: bacteria that live in the gut tend to induce strong immune responses in the gut-associated lymphoid tissues (GALT), including a good IgA response. Thus, bacterial vectors that express the antigens they are carrying in the gut would generate the type of immune response required to combat the enteric bacteria and viruses that cause diarrhoeal diseases. At the time of writing (2003) no bacterial constructs have been licensed for mass vaccination in humans, but Ty21 a is being tested as a vector for a variety of bacterial and other antigens, and the first human trial of HIV genes in a bacterial vector has begun.
Finally, an interesting development has been the insertion of single genes encoding critical antigens from bacteria and viruses into plant genomes, including those of potatoes and tomatoes. The antigens are expressed in the plant, which can then be eaten with the aim of inducing an immune response in the gut! The first trial of an oral plant vaccine against hepatitis B virus using genetically engineered potatoes began in the USA in 1999. If it turns out to be protective, it would have a significant effect in developing countries where viral hepatitis is a major health problem. Growing anti-HBV potatoes for local consumption would be a cheap and effective way of protecting the population.
Activity 15
What could limit the efficacy of oral plant vaccines?
Answer
Most proteins in foods are broken down by digestive enzymes in the stomach and intestine, so the critical antigens may be destroyed before they can elicit an immune response. Even if they survive digestion, most people do not usually produce immune responses to antigens in foods, perhaps because food antigens do not induce costimulatory signals for T and B cells.
Figure 7 summarises the current gene-based approaches to vaccine development.
In addition to the pragmatic question of whether a genetically engineered vaccine will induce protective immunity, there is a debate as to whether it is safe to produce such vaccines at all. The arguments are ranged along lines that have been well-rehearsed in more general concerns about genetic engineering. Critics think that altering infectious agents – albeit harmless ones – could produce pathogenic reversion, or further unplanned recombination of the vaccine vector with other bacteria or viruses to produce pathogenic strains. Proponents think that this risk can be made negligible by comprehensively disabling the vectors. The debate is difficult to resolve because although the risks and dangers of contracting an infectious disease are quantified, the risks and dangers from a new genetic vaccine are hypothetical. Other issues of vaccine safety are discussed in Section 8.