2.2 Partial pressure
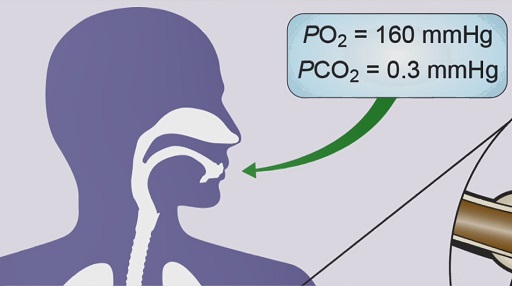
Pressure is an important factor in O2 and CO2 exchange in the alveoli. The pressure of each individual gas in the atmosphere is described as its partial pressure.
Partial pressure is calculated by multiplying the percentage of the particular gas in the atmosphere by the total atmospheric pressure. For example, O2 accounts for about 21% of the Earth’s atmosphere so the partial pressure of O2 (PO2) in the atmosphere is 0.21 × 760 mmHg = 160 mmHg. CO2 is present only in trace amounts, so the partial pressure of CO2 (PCO2) in the atmosphere is roughly 0.3 mmHg.
Question 4 Nitrogen
Nitrogen (N2) comprises 78% of the Earth’s atmosphere. What is the partial pressure of nitrogen (PN2)?
Answer
The PN2 in the atmosphere is 0.78 × 760 mmHg = 593 mmHg.
The difference in PO2 and PCO2 between fresh air and the blood drives the diffusion of O2 and CO2 down their respective concentration gradients, as described in Video 8.