2.3.1 Ion-channel receptors
Nicotinic cholinergic receptors are probably the best studied of all receptors, firstly because they are present throughout skeletal muscle, and secondly because there are plenty of natural and synthetic toxins that bind specifically to this receptor. Furthermore, the technique of patch-clamp electrophysiology has made possible the detailed characterization of the properties of individual ion channels (Figure 21a).
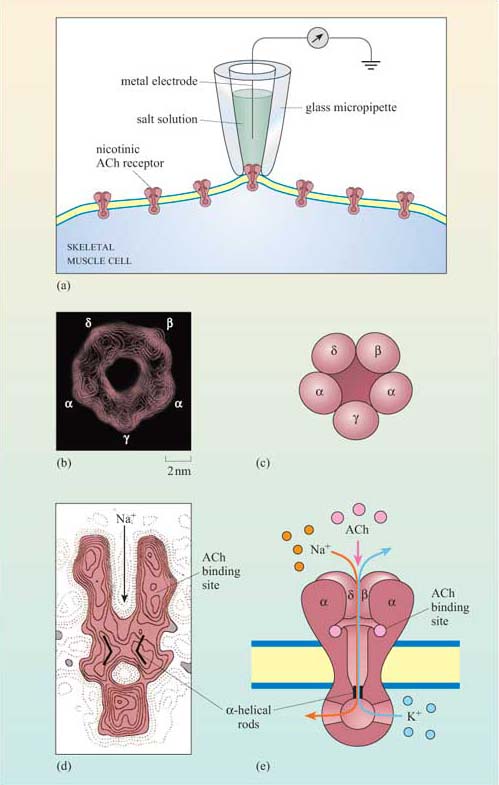
Nicotinic receptors are composed of five subunits (two α subunits together with one each of the β, γ and δ subunits), which assemble to form a pore in the membrane (Figure 21b–e). The pore can switch between an open and a closed state on binding of two molecules of acetylcholine to the two α subunits at sites within the channel (Figure 21). Although the channel alternates between an open and a closed state, binding of acetylcholine increases the probability of the channel being in its open state. When the channel is open, sodium ions flow into the muscle cell, using concentration and voltage gradients. The influx of positive charge due to the Na+ions inside the cell tends to locally neutralize the negative charge inside the cell (called ‘depolarization’). The channel is also permeable to K+ions, which exit the cell. However, the overall effect of the movement of ions causes the net charge inside the cell with respect to the outside to become more positive, and this is ultimately responsible for skeletal muscle contraction (Figure 7).