2.3.4 Recruiter receptors
Enzyme-associated or recruiter receptors also form dimers (or oligomers) on activation by their ligand, in a similar way to receptors with intrinsic enzymatic activity. Dimerization facilitates an interaction between the cell surface receptor (which lacks a catalytic domain) and cytosolic proteins with enzymatic activity. In the case of receptors that associate with tyrosine kinases (called ‘tyrosine kinaseassociated receptors’, the most common in this group), it is the non-covalently linked cytoplasmic tyrosine kinase which is autophosphorylated following receptor dimerization. Sometimes homo- or heterodimerization is not sufficient for receptor activation, and activation may follow oligomerization (clustering of several receptors on the membrane) or require membrane-bound co-receptors (structurally unrelated receptors necessary for signal transfer), which may even be RTKs. The end result of the multiplicity of activation combinations for these receptors is that it allows a refinement of signal specificity and diversity, as different downstream effectors are recruited depending on the ligand–receptor complex.
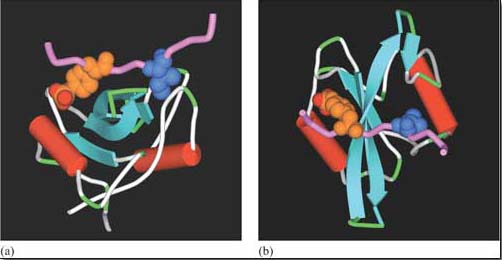
The Src family of tyrosine kinases are the biggest group of kinases that are recruited by tyrosine kinase-associated receptors. One example is Lck (Figure 26). In immune reactions, lymphocyte activation brings together T cell receptors (Figure 5) and other receptors (called ‘CD4’ or ‘CD8’ depending on the activated lymphocyte), which are associated with Lck. Clustering of receptors on the cell surface then results in the tyrosine phosphorylation of the T cell receptor by Lck and activation of downstream signalling pathways. Lck is also very adaptable. As well as associating with CD4 and CD8 receptors, it can also be recruited by means of its SH2 domains to other activated tyrosine kinase (or associated) receptors, thereby strengthening and propagating the signal.