3.3.1 Phosphatidylinositol 3-kinase (PI 3-kinase)
Members of this family of lipid kinases usually have two subunits: one is a catalytic subunit with a lipid kinase domain and the other is a regulatory subunit, which contains two SH2 domains and a SH3 domain (p 85 PI 3-kinase in Figure 13).
What will the SH2 domains of the regulatory subunit enable PI 3-kinase to do?
They will enable PI 3-kinase to bind to proteins containing a phosphorylated tyrosine residue within a specific motif. In this way, PI 3-kinase is targeted to the membrane when required, by binding to phosphotyrosine residues on activated RTKs.
The preferred substrate of PI 3-kinase is PI(4,5)P2. However, this kinase also phosphorylates PI and PI(4)P. It is the phosphorylation at the 3 position by PI 3-kinase that makes the molecule active in a signalling context. Thus, the two main products, phosphatidylinositol 3,4-biphosphate (PI(3,4)P2) and phosphatidylinositol 3,4,5-triphosphate (PI(3,4,5)P3), are both active signalling molecules because they are recognized by PH domains in other proteins (Figure 31). In contrast, PI(3)P is not an active second messenger.
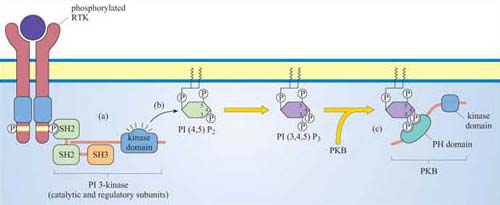
What distinguishes PI(3,4)P2, PI(3,4,5)P3 and PI(4,5)P2 from each other? Which are substrates for PI 3-kinase?
These molecules differ in their phosphorylation state. PI(3,4) P2 is phosphorylated on carbons 3 and 4, PI(3,4,5)P3 on carbons 3, 4 and 5, and PI(4,5)P2 on carbons 4 and 5. Only PI(4,5)P2 is a substrate for PI 3-kinase, whereas the two others are products.
How does the activity of PI 3-kinase influence the localization of signalling proteins?
By virtue of its catalytic activity, PI 3-kinase generates PI(3,4)P2 and PI(3,4,5)P3, which serve as plasma membrane docking sites for PH-containing proteins.
Proteins differ in their affinity for binding to either PI(3,4)P2 or PI(3,4,5)P3, depending on the interacting PH domain. One signalling enzyme that utilizes the membrane docking sites generated by PI 3-kinase is protein kinase B (PKB, also known as Akt), which thereby becomes an accessible substrate for an upstream kinase, PDK1 (not shown in Figure 31). PKB is a serine–threonine kinase, principally involved in mediating survival signals. Another important target is phospholipase C (PLC), which binds to PI 3-kinase substrates at the membrane via its PH domain (see below). The docking sites for PH domains are, as with all signalling components, temporary; specific inositol phospholipid phosphatases ultimately remove the phosphate from the 3 position of the inositol ring.