5.4 The medical chemistry strategy
The strategy adopted was to start from first principles. Since prazosin is an alpha1 antagonist, it must compete with the agonist, noradrenaline, for the alpha1 receptor. So the Pfizer scientists decided to compare the structures of the two molecules and look for common structural features that might provide an insight into the way in which they bind to the receptor. One important observation was the fact that, at physiological pH, both compounds would be protonated, noradrenaline at the terminal nitrogen and prazosin at N-1. They then postulated that one active part of the receptor was a polar entity such as a carboxylate group. If this was situated equidistant from the protonated N-1 in prazosin or the nitrogen atom in noradrenaline, so called charge-reinforced hydrogen bonding could take place equally well for both molecules. This model is shown in the reprint as Figure 5. This hypothesis indicated that the substituted quinazoline subunit (Figure 1 below) was an essential structural component in the prazosin series for specific binding to the alpha1 receptor.
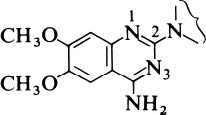
The next step was to investigate the effect of changing the structure of the rest of the molecule. The Pfizer scientists took a series of compounds, each containing the substituted quinazoline sub-unit, but with a variety of substituents attached at the 2-position, and tested them for alpha1 receptor affinity. This did not involve very much synthesis, since Pfizer maintains a chemical repository where every compound made in their laboratories is kept. These samples are catalogued before storing them in what is effectively a compound library for future reference. To a pharmaceutical company this is as valuable a resource as the more conventional document library. Details of each and every compound, including the structure, are then entered into a computer file. This ensures that, when required, retrieval both of the information and of the compound itself is very straightforward. So initial testing of the hypothesis simply involved searching the compound library for compounds containing the required diamino-quinazoline group and screening them for alpha1-blocking activity.
Several compounds containing the crucial substituted quinazoline group were tested both for alpha1 and for alpha2 activity. The 2-substituents covered a wide range, from the simple amino group through complex hetero-cycles.
The tests vindicated the hypothesis completely: none of the compounds showed any alpha2 activity but all had some degree of alpha1 affinity. The results are shown in Table 2. The figures in the first column are related to the concentrations of the compounds necessary to displace a radioactive ligand from the alpha1 receptor, so the smaller the number the more active the compound. All the compounds illustrated, except for 3 and 4, are highly active. But in vitro measurements of alpha1 affinity are only a guide to the effectiveness of the compounds as anti-hypertensive agents in vivo. The stars in the second column indicate how effective these compounds are in lowering blood pressure in rats after oral administration. These results show that the original compound, prazosin (10), still seems to be one of the most effective anti-hypertensive agents. So how to proceed?
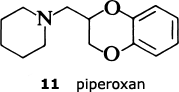
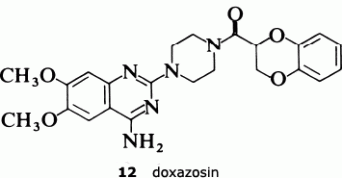
The results have confirmed the need for the substituted quinazoline subunit. The presence of a large substituent attached at the 2-position seems to increase activity and to improve the effectiveness in reducing blood presssure. The main goal in trying to develop a second-generation anti-hypertensive agent was to increase the duration of action so that the drug only needed to be administered once a day. The screening tests indicated that the furan ring in prazosin was particularly beneficial in this regard, and so the Pfizer scientists decided to look for an alternative oxygen heterocycle. They knew that compounds such as piperoxan (11) containing a benzodioxan group were also alpha-blockers. So Simon Campbell and his colleagues decided to investigate the effect of incorporating this group.
The result was doxazosin (12), a drug which only needs to be administered once a day and which, having passed through all the necessary toxicological evaluation and clinical trials, is now marketed as an anti-hypertensive agent.
We hope that this introductory video has demonstrated that research in the pharmaceutical industry is a complex and multidisciplinary process, and that progress is more often made by gradual advances rather than by sudden breakthroughs. There is no guarantee of success and thousands of different compounds may be examined in the search for the few that have the desired pharmaceutical activity. But above all, we hope it has shown the central role of organic chemistry, and organic synthesis in particular, in the discovery of pharmaceutically active compounds, and that while serendipity, or chance, often plays a part, the development of a successful new drug is increasingly the result of rational design.
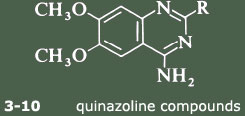 | |||
|---|---|---|---|
| Compound No. | R | Binding affinity/nmol l−1* | Effectiveness at reducing blood pressure in rats† |
| 3 | 190 | ||
| 4 | 33 | ||
| 5 | 3.4 | ||
| 6 | 2.5 | ||
| 7 | 2.0 | ||
| 8 | 1.5 | ||
| 9 | 1.0 | ||
| 10 | 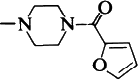 | 0.2 | |
