2 Molecular engineering
2.1 Understanding the polymer state
It was the pioneering scientific work of Hermann Staudinger in the early part of the twentieth century which led to an understanding of the polymer state at an atomic and molecular level. Until then, plastics and rubbers had been developed from naturally occurring substances or discovered during routine synthesis. His research laid the basis for all subsequent discoveries and their commercial development. In essence, he realised that polymers were large molecules built up by the repetition of small chemical units, known as repeat units, to create linear chains. More complicated structures consisted of linear chains with branches, and crosslinked molecules were effectively a single macromolecule of almost infinite molecular mass.
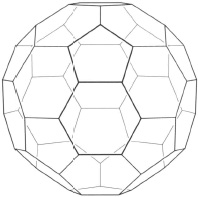
The development of entirely new polymer structures relies on a thorough understanding of the various ways in which non-metal atoms can be manipulated into chain structures, limited only by their valency and bonding properties. This subject is known as molecular engineering, and a glossary of terms in the subject is shown in Box 2. Owing to the versatility of carbon, it is this element more than any other which has been exploited in molecular engineering. Even now, fundamental discoveries about carbon are still being made by research scientists. Witness the discovery and development of fullerenes, molecules in which carbon atoms are linked together into closed three-dimensional structures such as balls and cylinders (Figure 15).
Box 2 Structural and bonding terms
aliphatic: a term used to describe non-aromatic carbon compounds, so includes both saturated and unsaturated linear, branched or cyclic compounds or structures.
alkane: a carbon compound where all the carbon-carbon bonds are single and saturated (C—C), like ethane and propane (also paraffins).
alkene: any organic compound containing a carbon-carbon double bond (C=C), like ethene (ethylene) or propene (propylene) (also olefins).
alkyne: any compound with the carbon-carbon triple bond (C≡C), like ethyne (acetylene).
aromatic: any structure possessing a benzene ring or higher derivatives such as naphthalene, and also heterocyclic rings as in polyimides.
asymmetric carbon atom: a saturated carbon atom where the four substituents are all dissimilar, so giving rise to stereoisomerism.
branched polymer: a linear chain to which are attached side chains.
catenation: the tendency of atoms of an element to link together to form chains.
cis: an isomer of a double bond in which two similar substituents are on the same side of the bond (inverse of trans isomer).
crosslink: any kind of tie between chains, whether covalent (as in vulcanized rubber) or based on secondary bonds (as in TPEs).
copolymer: chains in which there are two or more different types of repeat unit.
configuration: the structure of a chain fixed by covalent bonds. conformation: the structure of a chain determined by intramolecular rotation.
covalent bond: the strongest bond between atoms, where the outer electrons are shared between the participating atoms (also called primary bond, chemical bond).
homopolymer: a chain composed of identical repeat units.
hydrogen bond: a secondary bond type, between hydrogen and nitrogen or oxygen; important in nylons, polyurethanes, cellulosics and nucleic acids (e.g. DNA) as well as in small molecules such as water.
intermolecular bond: any bond between molecules.
intramolecular bond: any bond within a particular molecule.
ionic bond: any bond in a molecule where the attraction between the atoms is electrostatic, the electrons being passed from one atom to another, so that one part is cationic (+ve charge), the other anionic (−ve charge).
isomerism: phenomenon where polymers or small molecules have the same chemical formula, but different structures.
random coil: conformation adopted by single polymer chain where each repeat unit is randomly placed relative to its predecessor or successor.
stereoisomers: polymers or small molecules with the same formula but which differ in the spatial arrangement of the substituent atoms, as in tactic polymers; caused by presence of an asymmetric carbon atom.
tacticity: type of isomerism found in vinyl polymers, and caused by an asymmetric carbon atom in the main chain; as in isotactic, syndiotactic and atactic polymers.
van der Waals bond: type of weak, secondary bond between atoms and molecules; occurs widely in organic compounds or polymers, especially between distinct molecules.
Realisation of theoretical ideas on the structural possibilities, however, has always presented practical problems of synthesis, availability and cost of raw materials, and ingenuity in finding application in real products which will sell in the open market. Thus, as of 1997, fullerenes remain a laboratory curiosity in terms of commercial application. Nevertheless, it is only a matter of time and development before such compounds and their isomers are of practical benefit and use.
