2.3.1 Structural isomerism
In the saturated hydrocarbons, whose structural formulae are shown in Figure 16, it is not possible to form distinct isomers with just three or less carbon atoms linked together. There is only one way in which one carbon and four hydrogen atoms can be linked together, the single compound being methane, CH4. A similar situation holds for ethane, C2H6 and propane, C3H8. But with butane, two possible structures can be formed: n-butane, which has a linear structure, and iso-butane, where the central carbon atom is linked to three adjacent carbons rather than one (Figure 16). Their physical properties are slightly different, for example their boiling points differ by 10 °C, but otherwise they are very similar compounds. Drawing the possible structures (or using structural models) of the isomers gives 3 isomers for pentane, 5 for hexane, 9 for heptane, and so on. As the number of possible structures increases, their properties diverge: the boiling points of the heptanes range over 20 °C and the melting points by no less than 110 °C. The chemical properties differ too: witness the different combustion behaviour of n- and iso-octane in petrol engines. The straight chain isomer causes ‘pinking’ during combustion, while the branched chain burns smoothly in the engine chambers (hence the familiar ‘octane rating’ for petrols).
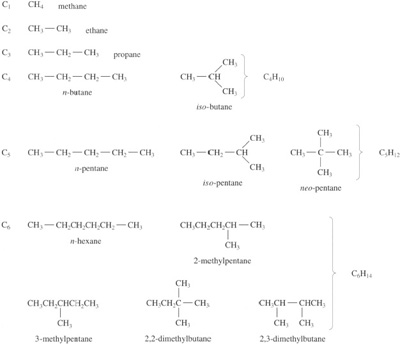
The increase in isomeric structures is so rapid that at C30, there are no less than 4 111 846 763 theoretically possible compounds! So with even the lowest molecular mass polyethylene, there is an almost infinite number of isomers. Fortunately, the situation is simplified enormously by the way polymerization occurs and in fact there are relatively few chemically distinct polyethylenes. As we shall see later, the concept of a distinct molecular formula is redundant with most commercial polymers since chain lengths are very variable even within a single sample, but the idea of branching is important for polyethylene in particular.
Box 3 Spectroscopy and polymer analysis
Although information on the polymer or polymers used in a specific product may be provided on the packaging or the product itself, it is often absent, and so some way of analysing the material is needed for identification. It is usually provided by spectroscopic analysis, where a small sample is exposed to electromagnetic radiation and the absorption by the material of specific frequency bands is usually diagnostic of the functional groups present in the polymer. UV or ultraviolet spectroscopy is most useful for detecting aromatic groups in polymer chains, such as the side chain benzene rings in polystyrene. It is infrared or IR spectroscopy which is most useful, however, for analysis of polymers. The method essentially detects radiation absorbed by different bonds vibrating within the chains. Real chains are always moving and vibrating in polymers (unlike the static models for chains shown in this text!), and their vibration frequencies depend on the sizes and masses of the atoms which the covalent bonds link together. There are also different ways in which the bonds can vibrate: they can stretch along or bend (rock) about the bond axis, for example:
Stretching involves a higher energy input than bending, so radiation is absorbed at a higher frequency than rocking. Since frequency, v is inversely proportional to wavelength, λ (from the formula λ = c/v, where c is the velocity of light), C—H bonds will absorb IR radiation at a lower wavelength when stretching compared to rocking. Of functional groups, the carbonyl group (C=O) is especially important for diagnosis of the polymer since it occurs in nylons and polyesters. Bond stretching occurs in a very narrow band centred at about 6 μm wavelength, so when a strong peak is detected at this position in the IR spectrum, it is likely that the carbonyl group is present in the polymer. It doesn't necessarily follow that the polymer is a nylon or polyester, however, because such groups can be present even in polyethylene chains as a result of oxidation. Band 3 of the AV cassette gives an account of a forensic investigation concerning a PE product which fractured as a result of oxidation and chain degradation. IR and UV spectroscopy were critical tools in the determination of the causes of the failure of the product.
