2.3.2 Chain branching
A germ of the idea is shown by the formulae for 2- and 3-methylpentane in Figure 16. A single methyl group (CH3—) can occur in two different positions along an essentially linear carbon-carbon chain. The methyl group is a very simple kind of branch along the chain, and it is easy to extend the idea to much larger molecules. Thus LDPE is a polymer based on a linear backbone chain with the repeat unit [CH2CH2], but is in addition branched with very long chains at infrequent points along the main chain (about 1 in 1000), as shown in Figure 18. Branching is caused during polymerization at high pressure by growth sometimes starting from an initiation point in a chain rather than at the end. An alternative way of making polyethylene is at low pressure using a special catalyst, and this usually results in a highly linear chain without branching (HDPE).
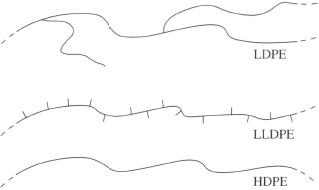
However, it is easy to polymerize a mixture of ethylene with a higher alkene such as hex-1-ene, so that the new units co-polymerize together to form a chain where a certain proportion of the chains have tails or short branches along the linear sequence (Figure 18). This and similar copolymers are generically part of the polyethylene family, and are known as LLDPE, short for linear low density polyethylene.
This kind of structural variation is important because it affects the properties of the polymers, as their names indicate. Thus branches along the chain hinder crystallisation of the chains, resulting in a less dense and lower modulus material. LDPE typically has a density of 0.92 Mg m−3 while HDPE has a higher density of 0.96 Mg m−3. (Note that these density values are numerically identical to those expressed in g cm−3.) Intermediate grades, MDPE, are other important relatives, finding wide application in gas pipes, for example.
