2.3.3 Geometrical isomerism
A second type of isomerism occurs with diene monomers, and is present in both NR and butadiene rubbers (BR). It occurs because the single double bond in the final polymer can exist in two ways: a cis form and a trans form. The repeat unit shown in Table 3 for NR does less than justice to the two-dimensional structure of this material (Figure 17). In this planar formula where the bonds are shown in their correct orientation to one another, the base polymer in natural rubber can be seen to be m-polyisoprene, where the pendant methyl group appears on the same side as the lone hydrogen atom. The two parts of the chain in which this single repeat unit sits, lie on the opposite side of the double bond.
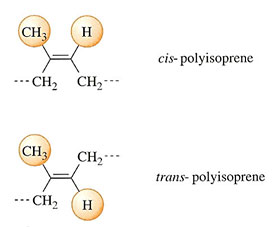
But there is an alternative structure with exactly the same formula: the polymer is gutta percha, and its structural formula is shown below that of NR in Figure 19. Here, the two parts of the chain are on opposite sides of the double bond, giving the overall chain a zig-zag appearance. It is also a naturally occurring polymer, but has quite different properties to natural rubber. It is a highly crystalline and rigid material without the long-range elasticity characteristic of an elastomer. It was used formerly as an electrical insulator, but has now been largely superseded by synthetic plastics.
