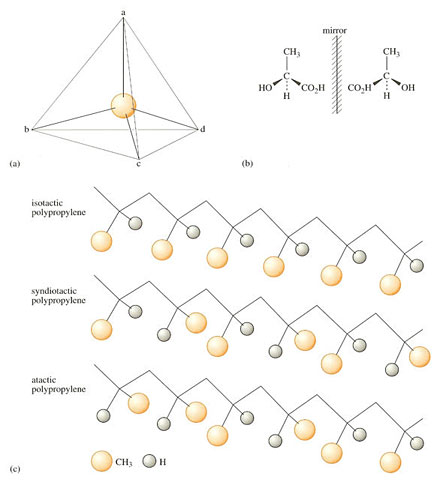
2.3.4 Stereoisomerism
A final type of isomeric variation occurs as a result of the three-dimensional structure of some polymers. It is possible because a four-valent atom like carbon can exist in two different forms when the subsidiary groups or atoms attached to the carbon are all different. The carbon atom is then known as an asymmetric carbon atom. A very simple example of the phenomenon is the structure of a small molecule, lactic acid. As Figure 20 shows, it can exist in two forms which are mirror images of one another. One of the two possible compounds, laevo- (standing for left-handed), or l-lactic acid occurs in muscle after vigorous, anaerobic exercise and causes muscle cramp. It is a good example of stereoisomerism in a small molecule, a feature it also shares with a large number of biological molecules, as well as some polymers.
The carbon atom in a vinyl polymer to which is attached the pendant side group (i.e. every alternate carbon atom in the main chain) is another example of an asymmetric carbon atom. It gives rise to tacticity. When the zig-zag chain is written with the plane of the chain in the plane of the paper, there are three ways in which the position of the pendant side group can exist. The methyl groups in polypropylene, for example, can occur all on one side (isotactic), on alternate sides (syndiotactic) or placed at random (atactic). These possibilities are shown in Figure 20(c). The properties of each type of polymer are quite different to one another, primarily because isotactic and syndiotactic PP have ordered chains and so can crystallise, but atactic chains are quite irregular and cannot crystallise. Isotactic PP is the common form of the commercial material, although atactic PP is used as a binder for paper for example. Syndiotactic PP has recently become available commercially (1997) being made using a new family of catalysts, known as metallocenes (see Section 4.2.5).