2.5.2 Polymer families
But how do small changes in chain configuration for a given family of polymers affect their properties? A very clear example of slight changes in the repeat unit structure is exhibited by polyamides, polyesters and polyurethanes. They are all polymers linked together by a particular kind of functional group, which gives the name to each family. Their backbone chain may either be aliphatic or aromatic in nature, although here we'll only be examining trends for the aliphatic polymers. One way of looking at their structure is to regard them as comprising a polyethylene chain into which is inserted the functional group of interest.
They are crystalline polymers which show a distinct melting point (Tm) and we are interested in the effect on Tm of changing the chain length of the sectors between functional groups. Since such polymers are made from two separate monomers (cf. the repeat unit in nylon 6,6 of Table 3), only one of the sectors will be varied. The melting temperatures are shown in Figure 11 as a function of increasing sector chain length. The constant melting point of HDPE is shown on the same figure for comparison.
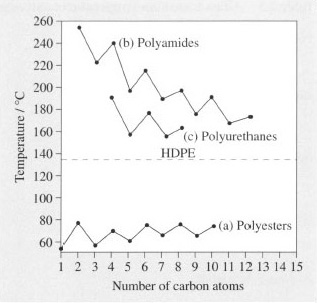
The first point of interest is that the polyamides and polyurethanes always have melting points above that of HDPE, an effect due to hydrogen-bonding between adjacent chains. This is a secondary kind of bonding between hydrogen and oxygen or nitrogen, which occurs in water and ice as well as many natural polymers. Hydrogen bonds occur between the functional groups on neighbouring chains and effectively tie the chains together in a weak kind of crosslink. This means that the crystalline chains are held together in an energetically more stable conformation than in polyethylene itself, so a higher temperature is needed to melt or decompose the crystals. Hydrogen bonding is absent from polyesters, so the crystals actually show a lower melting temperature than PE because the functional groups make for a less well packed, and hence less stable, structure.
The effect of increasing the number of carbon atoms in the intervening PE chains in the amides and urethanes is similar, lowering Tm in a regular way and approaching the limiting value for HDPE. The decrease is not smooth, however, values oscillating about the line. This effect is caused by packing effects in the crystal structure, odd numbers of carbon atoms fitting together less easily than even numbered chains. With the polyesters, the packing effect still creates oscillations, but increasing chain length has a minimal overall effect on melting temperature.
Such trends are important in choosing specific grades for specific end products, or providing designers and manufacturers with a set of polymers with slightly different thermal properties but whose other properties remain the same. For example, nylon 6 is a high melting polyamide (Tm= 225 °C), and it might be desired to lower this temperature for saving energy during processing. Nylon 11 with a Tm of circa 180 °C might then be good choice of material. The hydrogen bonds in the chain still confer the useful property of solvent resistance, for example, and this resistance is unlikely to be affected by lengthening the chain sector between functional groups.

|
