4.2 Chain and step growth
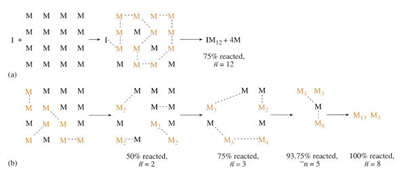
There are two basic ways of making chains. The first is to activate a small number of monomer units M which then successively consume other monomers. This mechanism is known as chain growth and is shown schematically in Figure 36 (a), where a monomer unit is activated by initiator I and forms a chain very quickly. After 75 per cent of the monomer molecules have reacted in this case the degree of polymerization n = 12.
The second mechanism is to activate all the monomer M present so that larger and larger fragments are formed throughout the material in the reaction vessel. This is known as step growth and is shown in Figure 36 (b). Since all the monomer molecules can interact with one another, the length of the chains increases more slowly during the course of polymerization. Thus at 75 per cent completion, the average degree of polymerization (
![]() ) is one quarter of that for a chain growth reaction. All double-bonded monomers, like the vinyls and dienes, polymerize in a chain growth mechanism whereas monomers with functional end groups react in a stepwise fashion, for example, terephthalic acid and ethylene glycol (Table 5) which form PET.
) is one quarter of that for a chain growth reaction. All double-bonded monomers, like the vinyls and dienes, polymerize in a chain growth mechanism whereas monomers with functional end groups react in a stepwise fashion, for example, terephthalic acid and ethylene glycol (Table 5) which form PET.