4.3.1 Initiation
Initiation is the mechanism which starts the polymerization process. Vinyl monomers are quite easily polymerized by a variety of activating methods. Styrene, for example, can be converted to solid polymer simply by heating, and ultraviolet light can have exactly the same effect. Usually, however, an activating agent is used. This is an unstable chemical which produces active species that attack the monomer. A good example is benzoyl peroxide which splits up when heated:
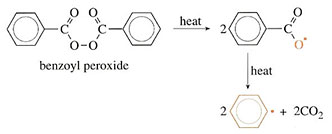
The formulae of the products are written with a dot alongside to show that they are free radicals. A free radical is a molecule in which there is an unpaired electron. This free radical is very reactive and will attack monomer molecules when introduced into a polymerization vessel. Thus, as benzoyl peroxide is added to styrene (a reaction used with GRP), the peroxide splits to make free radicals, which react as follows:
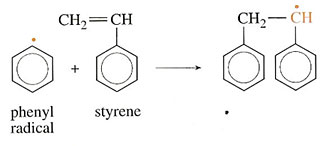
The net result is that the reactants have been linked together but the product is still a radical and so is capable of attacking further monomer molecules. In each instance the attack will lead to a larger molecule but the free radical will be preserved. The reaction is referred to as free radical polymerization.
Free radicals are not the only way of initiating reactions. Charged molecules can often exert the same effect. Ethyllithium, for example, is a relatively unstable molecule which can dissociate to form an ion pair:

Styrene can also be polymerized by this compound:
![]()
This mechanism is called anionic polymerization. The next two sections refer to free radical polymerization. Ionic polymerization will be discussed in Section 4.2.4.
