4.3.4 Ionic polymerization
Free radicals are indiscriminate in the compounds they attack, and their non-selective nature in polymerization reactions leads to problems such as chain branching and transfer which affect the structure of the polymer produced. Anionic polymerization overcomes many of these problems.
A typical commercial (but also see Box 8) anionic reaction is the polymerization of styrene using butyllithium, C4H9Li, in an inert solvent such as n-hexane. Termination does not occur by polymer-polymer interaction but by reaction with small molecules such as water:
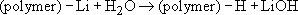
This type of polymerization gives rise to very sharp molecular mass distributions because transfer processes are absent. If the solvent is extremely pure, the polymer chains will still be active after all the monomer has been consumed. Such activated systems are known as living polymers and it is possible to continue feeding monomer into the reaction vessel without killing the living chains. The degree of polymerization is simply

Since the chain ends are relatively few in number only a very small amount of water need be present to kill the polymer, and so all ingredients must be rigorously purified. Paradoxically, it is easier to conduct the reaction on an industrial scale than in a laboratory flask. An important source of contamination is the sides of the reaction vessel itself. Since the surface area of a sphere (assuming a spherical reaction vessel of radius r) increases as r 2 while the volume increases as r 3 the problem of surface contamination will be less serious in large, industrial reactors than in laboratory-scale reaction vessels.
Just as negatively charged initiators can be used to start polymerization, so positively charged species can initiate chain growth. The most important commercial operation is the polymerization of iso-butylene giving butyl rubber using aluminium chloride. The reaction conditions are unusual in that high molecular mass polymer is formed very rapidly at very low temperatures (−100 °C for example).
Box 8 Superglue
A more familiar example of anionic polymerization occurs when you use cyanoacrylate liquid (”superglue’) to stick a broken pot together. The monomer is
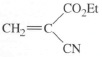
and being a small molecule, has a very low viscosity (Et is the abbreviation for the ethyl group −C2H5). This is an important property for adhesion, because it means that the liquid when applied to the broken pot will penetrate even the finest cracks in the fractured surfaces. Such surfaces will normally already be very slightly wet with water from the atmosphere (a monomolecular film is enough), and the monomer will start to polymerize anionically. The anion is supplied by the small amount of hydroxyl ions present in water:
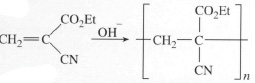
Reaction is very fast, and since this is a chain growth mechanism, high molecular mass polymer is created very rapidly. You might, if unlucky, already have experienced this effect if you accidentally spilt the monomer on your fingers and they made contact! The sweat present there is more than enough to initiate polymerization. The good news is that since the polymer is thermoplastic, there are solvents available for swelling or dissolving the bond and so releasing your fingers. Termination occurs when no more monomer is present, so that all the liquid monomer present at the interface between the two parts of the pot becomes solid polymer. The polymer chains pass from one broken surface to the other, so adhesion is excellent, and strength will be maximised. A range of such cyanoacrylate monomers is available now with varying rates of polymerization and modes of initiation (e.g. thermal or pressure initiation), as well as grades which react without air or water being present, the so-called anaerobic superglues. These adhesives have slightly different substituents, so affecting the way the monomer behaves during reaction.
Self assessment question 6
An anionic polymerization is initiated with a solution containing 0.1 moles of n-butyllithium in 100 ml of n-hexane. Initiator (1 ml) is added to a litre of hexane solution containing 1 mole of styrene monomer. At the end of the reaction, another mole of styrene is added and the reaction is terminated with water. What is the molecular mass of the polystyrene extracted from the solution?
Answer
The degree of polymerization can be calculated from Equation (17)

since the volume of the reaction can be assumed to be constant. As the polymer will still be living at the end of the first phase of reaction, the total number of moles of monomer is simply 1 + 1 = 2 moles of styrene. The initiator solution comprises 100 ml of hexane containing 0.1 moles of butyllithium. So 1 ml added to the reaction vessel will contain
0.1 × 10-2 = 10-3 moles of initiator
Hence
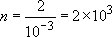
The molecular mass can be simply determined from Equations (1) and (2), the atomic masses of carbon (12) and hydrogen (1) and the repeat unit structure
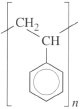
So MR = (8 × 12) + (8 ×1) = 104
Hence M = 208 000
