5.6.3 Conformation and crystallinity
If there are key connections between the chain configuration and crystallisation, you might also expect some more subtle effects from rotation about chain bonds. After all, polymer chains must be able to twist into the regular conformation demanded for crystal structures (Figure 57(a)). And what influence will rotation have on the precise conformation adopted by the chain?
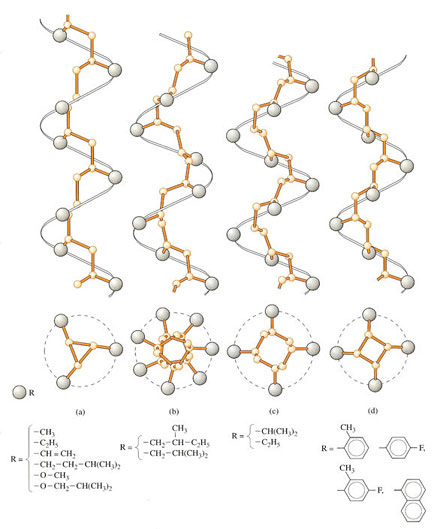
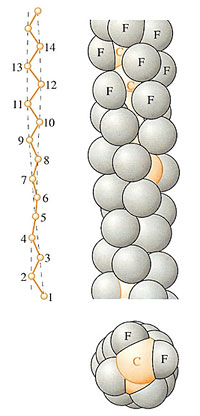
Polyethylene crystallises into the most stable conformation represented by the linear zig-zag, but when substituents are present, as in propylene, then there is substantial steric hindrance from the pendant methyl group at each alternate carbon atom. In polypropylene and related polyolefins, the chains adopt a helical conformation (Figure 57) where the extra side groups are accommodated on the outside of the helix by regular twisting of the whole chain. And as you might expect, the larger and longer the side group, the larger the diameter (Figure 57(b) and (c)). With large aromatic side groups, the pitch of the helical conformation grows (Figure 57(d)), but the diameter actually shows a slight decrease. A similar effect occurs in PTFE, –[CF2–CF2,]– where the fluorine atoms present on all the carbon atoms, pack neatly together into the helix (Figure 58). The figure is based on a so-called space filling rather than outline model (as shown in the previous figure), so that the true atom sizes are taken into account. This is useful, because it demonstrates that no part of the backbone chain itself is visible. It is thought that this is one of the reasons for the exceptionally low coefficient of friction of PTFE μ∼0.04), because fluorine has extremely weak van der Waals bonds with others around it, whether other fluorine atoms or foreign atoms at an interface.
There is also the problem of rate of crystallisation. One might expect hindered repeat units to crystallise only slowly. This explains why polypropylene crystallises so slowly when compared with polyethylene.
