2.1 Metallic bonding
The attraction between the delocalised electrons and the positively charged nuclei is called metallic bonding. The metallic bonding is strong and occurs in all directions. Breaking this attraction is difficult, and this is the reason that metals have high melting temperatures.
Metals are good conductors of electricity and heat, because the free moving electrons facilitate the transfer of charge or heat through the material.
Figure 5 shows a simple electric circuit with a metal wire, a battery and a bulb. When the wire is connected between the positive and negative terminals of the battery, one end of the wire becomes positively charged and the other becomes negatively charged. This causes the electrons, which are free to move, to travel through the wire towards the positive terminal of the battery, where they are removed. At the same time the negative terminal supplies more electrons to the wire.
Within the battery there is a net flow of negative charge from the positive terminal to the negative terminal, which balances the flow through the wire, so that charges don’t continually build up at the battery terminals.
You may be familiar with the term ‘voltage’. The voltage of the battery can be considered as the ‘push’ exerted on electrons moving along the circuit and the flow of negatively charged electrons in the wire constitutes the electric current. Note that in Figure 5 the arrows refer to the flow of electrons. By convention, the direction of the electric current is in the opposite direction, from the positive terminal towards the negative terminal of the battery.
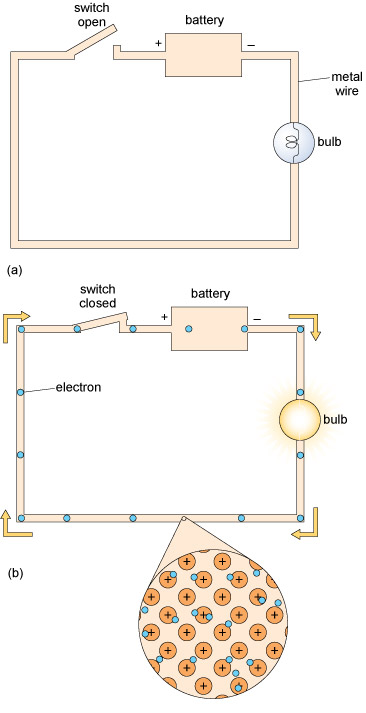
When will the bulb light?
The bulb will light only when the circuit is closed and there are electrons flowing through it.
The battery voltage depends on the choice of chemicals inside it.
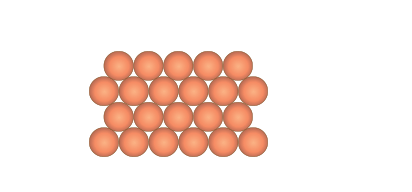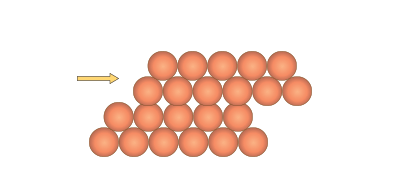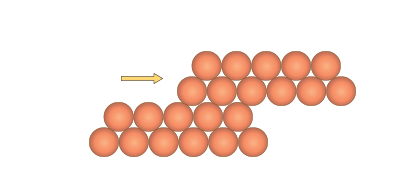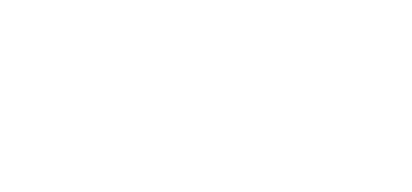
The delocalised electrons also explain other metallic characteristics such as malleability. The bonding occurs in every direction throughout the metal enabling atoms to roll easily over each other without breaking any bonds when stress is applied (Figure 6).