3.1 Practical 1 Flame tests
In this section you will investigate the presence of metal ions by recording the colour observed when heating a metal salt in a flame. You will be using solid samples of different metal salts. The metal chlorides you will be analysing are those of copper, lithium, potassium and strontium.
This practical activity will take 45 minutes.
You will perform the experiment in the virtual OpenScience Laboratory (the OU online laboratory for practical science).
What you need to do:
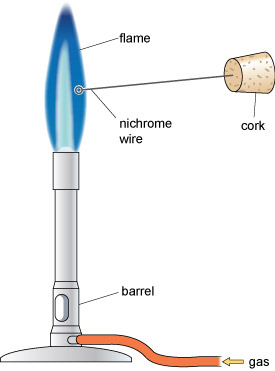
- 1. Turn on the gas, pick up the lighter and light the Bunsen burner, which should give a low yellow flame. Rotate the barrel of the burner so the air hole is open and the flame is blue.
- 2. One end of the nichrome wire is embedded in a cork for safe handling, and the other end has a small loop. Clean the loop of wire by dipping it into the small beaker containing a solution of hydrochloric acid.
- 3. Place the loop into the side of the blue flame, as shown in Figure 10. If the wire is clean it should make no difference to the colour of the flame. If the colour of the flame does change there is an impurity on the wire. Dip it again in the acid and return it to the side of the flame.
- 4. Dip the loop of the wire into the acid and then use it to pick up a few grains of a metal salt.
- 5. Place the loop in the side of the flame and note down the colour of the flame in your copy of Table 2.
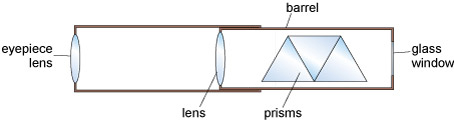
- 6. Use the hand spectroscope provided to separate the constituent colours present in the light and look at the emission line spectrum of the flames. Note down the description of spectra in your copy of Table 2. The hand spectroscope is a simple piece of equipment that houses a prism system in order to provide spectra from visible light (Figure 11).
- 7. Repeat this procedure and observe the flame colour given by the other metal salts. Record your observations in Table 2.
| Metal | Chemical symbol | Flame colour | Description of spectrum |
| Lithium | |||
| Copper | |||
| Strontium | |||
| Potassium | |||
| Sodium | Na | Yellow | Two bright yellow lines, one weaker blue line, one weaker green line and one weaker red line |
- 8. Now try looking at the colour of the flame obtained with the unknown mixtures of metals. Write down what you observed.
Follow the link to access the experiment. Instructions are also provided within the experiment, under ‘Help’.
Practical 1 Flame tests in the OpenScience Laboratory [Tip: hold Ctrl and click a link to open it in a new tab. (Hide tip)]
If you don’t know the contents of a mixture, are you able to identify the metals present using the flame colour?
It is not easy to identify the contents of a mixture based on the flame colour. Often one flame colour will dominate or a different colour is observed that could be the combination of two colours.
What spectrum would you expect to see if a mixture of lithium and copper salts is placed in the flame?
The spectrum of a mixture of lithium and copper will show red and orange lines from lithium and green and blue lines from copper.
Metals are added to the gunpowder used in fireworks to produce light of different colours when the powder burns. Which metal would you add to gunpowder in order to produce red light?
Strontium will produce red light.