4.1 Metals and life
Metals also play an important role in biological systems. Iron is essential for transporting oxygen in the blood and tissues. Some metals are part of biological structures: for example, calcium provides strength to our bones (Figure 13). Maintaining different concentrations of sodium and potassium inside and outside living cells is critical for body functions such as muscle contraction and heart function. The presence of metals (such as zinc) is also required for many essential enzymatic reactions (for example, the digestion of proteins).
How do we maintain adequate levels of metals in our bodies?
We acquire these elements from the food and water that we consume.
Metals normally occur at very low concentration in our bodies and are known as trace elements. At high levels metals may be toxic. In particular, metals such as mercury and lead can interfere with the structure of proteins and their effective function.
Nowadays, consumption of dietary supplements is very common; however, recommended daily allowances should be observed.
| Metal | Chemical symbol | Concentration/ppb |
| Aluminium | Al | 13 |
| Cobalt | Co | 0.2 |
| Chromium | Cr | 3.0 |
| Nickel | Ni | 5.0 |
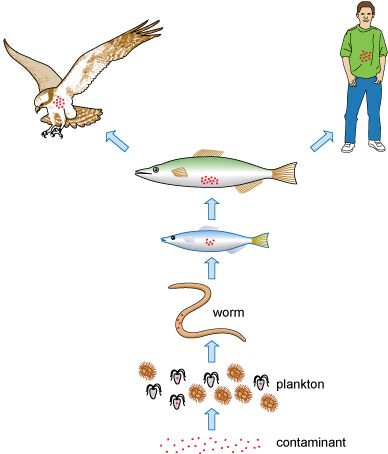
Metals may enter fresh and salty water through industrial waste, sewage and run-off. Microbes, plants and animals that depend on this contaminated water consume or absorb these metals. Over time the metals are concentrated within the food chain, in a process known as bioaccumulation (Figure 14). The concentration of metals in affected organisms is greater than was initially present in the water itself, as species consume greater quantities at each level and so the concentration increases up the food chain.