5.3 Order and chaos
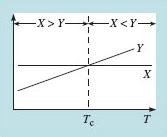
How can we explain a sudden switch of behaviour at a particular temperature? There must be two competing influences (say X and Y) that depend differently on temperature. Figure 23 indicates how a unique temperature (a so-called critical temperature, Tc) arises, at which the balance changes from X > Y to X Y
Now, if one influence is promoting order and the other is promoting chaos, it clearly matters which wins. Here, perhaps chaos is too strong a word – atoms in a liquid are more disordered than the same atoms locked together as a crystalline solid. Likewise, atoms in a gas are more disordered than those same atoms in the liquid state. The factor that promotes order in materials is the formation of cohesive bonds between neighbouring atoms, and the strength of the bonds changes only slowly with temperature. A tendency for disorder, on the other hand, seems to be a fundamental consequence of thermal energy.
Do you want to know more? Further details require an understanding of thermodynamics, and especially the concept of entropy, topics beyond the learning outcomes for this block, so I do not propose to delve deeper. What is important for now is that some characteristics of materials switch abruptly at a critical temperature, whereupon a change of phase occurs.