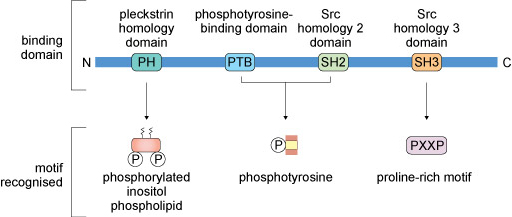
3.3 Functional domains in signalling proteins
Signalling proteins typically have conserved amino acid sequences (domains) that enable them to bind to other proteins, lipids or allosteric regulators. Some commonly encountered protein domains and the motifs or targets that they recognise are shown in Figure 11. The domains are often named because of a specific function. For example, the ‘phosphotyrosine-binding domain’ (usually abbreviated to PTB) enables its host protein to bind to another protein that has a phosphorylated tyrosine residue. Other domains are named because they have some sequence homology with the protein in which the domain was first recognised. For example, ‘Src homology 2 domain’ (SH2) is analogous to a phosphotyrosine-binding domain in the protein Src (pronounced ‘sark’). There are tens of different domains that direct proteins to bind a wide range of targets.
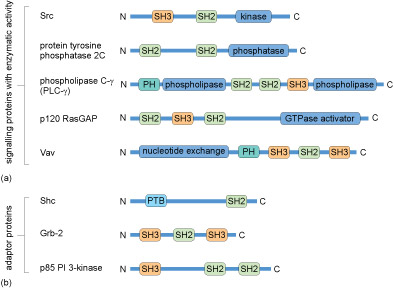
Many proteins contain several of these domains and can bind multiple partners simultaneously. Because of this multiplicity of binding domains, proteins can often form complexes to facilitate the flow of information through a signalling pathway. Linear representations of some signalling proteins and their binding domains are depicted in Figure 12. It is evident that these proteins all have at least two binding domains. All of the different types of proteins listed in Box 1 would have to possess some binding domains; otherwise, they would not be able to interact with upstream or downstream targets.
Activity 8 Protein-binding domains underlie formation of signalling complexes
Figure 13 shows a hypothetical signalling pathway that involves three signalling proteins and an adaptor protein downstream of a receptor; the motifs recognised by particular binding domains are indicated on the figure. Drag and drop the labels onto the unlabelled binding domains to complete the diagram (you can also scroll through the options and make your choices by clicking on or tapping a label field on the figure). You may find it helpful to refer to Figure 11.
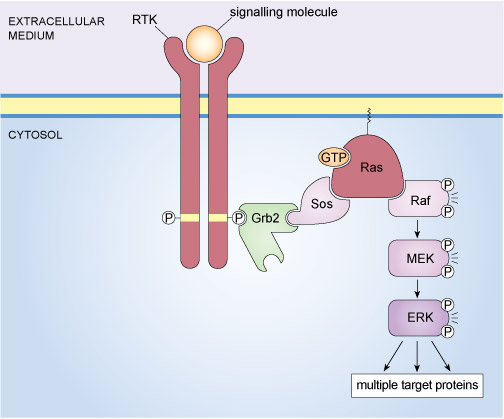
An example of a well-known signalling complex that is formed through specific protein interactions is the mitogen-activated protein kinase (MAP kinase) pathway illustrated in Figure 14. This signalling pathway operates in mammalian cells and is stimulated by the binding of growth factors to tyrosine kinase receptors. The final element of the MAP kinase pathway is a serine/threonine kinase called ERK (an acronym for extracellular signal-regulated kinase); however, there are several steps between the binding of a growth factor to its receptor and the activation of ERK. In essence, a signalling complex comprising a receptor tyrosine kinase (RTK), an adaptor protein (Grb2), a GEF (Sos), a monomeric G-protein (Ras) and three kinases (Raf, MEK and ERK) has to come together at the cell membrane in order for an extrinsic stimulus to cause a cellular response.
There are two isoforms of ERK MAP kinase, referred to as ERK1 and ERK2. Note, however, that since they have similar mechanisms of regulation, scientists don’t always distinguish between these isoforms and use the term ERK to refer to either or both.
The assembly of the components of the mammalian MAP kinase pathway begins with the SH2-containing adaptor protein called Grb2 (growth factor receptor-bound protein 2). Grb2 has no enzymatic activity and consists of little more than two SH3 domains flanking an SH2 domain (Figure 12(b)). Grb2 binds to an activated RTK via its SH2 domain. In turn, Grb2 associates with Sos by virtue of one of its SH3 domains. Sos (which is a GEF) stimulates the exchange of GDP for GTP on the monomeric G-protein Ras, thereby activating it. The active, GTP-bound Ras is then able to recruit the serine/threonine kinase Raf to the membrane, where it becomes activated through a complex series of steps that involves phosphorylation of one Raf molecule by another. Raf subsequently phosphorylates MEK, thus activating it. MEK then phosphorylates the MAP kinase ERK, which goes on to phosphorylate a range of target proteins, including transcription factors.
Signalling through MAP kinases is conserved across the animal and plant kingdoms. A comparison of MAP kinase pathways in various animal species and in plants reveals a similar organisation to the various MAP kinase pathways across species, with the MAP kinase pathways being activated by extrinsic signals that bind to membrane receptors and a cascade of three kinases that relay upstream signals to downstream effectors.
