1.2.1 Minerals
A mineral is a solid material, formed by natural processes and with a chemical composition that falls within certain narrow limits. Its constituent atoms are arranged in a regular three-dimensional array or pattern and because of this, minerals form crystals with characteristic shapes.
Although several thousand different kinds of mineral have been discovered, only a few are very common; for example, the mineral quartz, which forms many of the sand grains on a beach or in a desert. Because silicon and oxygen are the two most abundant elements in the Earth's crust, they are the main ingredients of common minerals, and these minerals are known as silicate minerals. They include quartz, whose chemical composition is silicon dioxide (formula SiO2), and a range of others containing additional common chemical elements. A common mineral that is not a silicate is calcite; it has the chemical composition calcium carbonate (CaCO3). Whereas most minerals are identified on the basis of physical characteristics (density, hardness, colour, etc.) calcite can also be readily identified with a chemical test. Calcite reacts with dilute hydrochloric acid, liberating bubbles of carbon dioxide (CO2) gas in a vigorous fizzing froth. The chemical reaction is written as
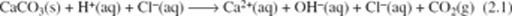
In words, this equation can be expressed as: solid calcium carbonate (calcite) plus hydrogen ions in the acid plus chloride ions in the acid gives calcium ions, hydroxide ions and chloride ions dissolved in solution plus carbon dioxide gas.
