1.3.4 Classifying igneous rocks
To classify (i.e. to name) igneous rocks, geologists use three pieces of information in combination – the grain size and the identity and proportions of the minerals present. The identification of minerals in a rock relies on recognising their particular distinguishing features. Such features include colour, lustre (the way in which light is reflected from the mineral's surface) and shape. The way in which certain minerals break apart along preferred planes, a property known as cleavage, can also be useful in identifying minerals because this property depends on the way that the mineral's constituent atoms are arranged. In some minerals the atoms are bound more strongly in some directions than in others, in which case there are natural planes of weakness present in the crystal. The mineral tends to break preferentially along these planes.
One mineral that shows this feature clearly is mica, a silicate mineral containing potassium (K), iron (Fe), magnesium (Mg) and aluminium (Al), together with hydroxyl (OH) groups. These compositional details needn't distract us, but the important point is that the potassium ions occur in layers, which separate sheets of more tightly bound silicon, oxygen and other atoms. Mica therefore splits apart parallel to the sheets, so has just one set of cleavage surfaces parallel to each other (Figure 5a). This is why mica forms platy or flake-like crystals, rather like the pages of a book. Other minerals can have two or three sets of cleavages (which intersect at characteristic angles) whereas others, notably quartz, have no cleavage and break irregularly.
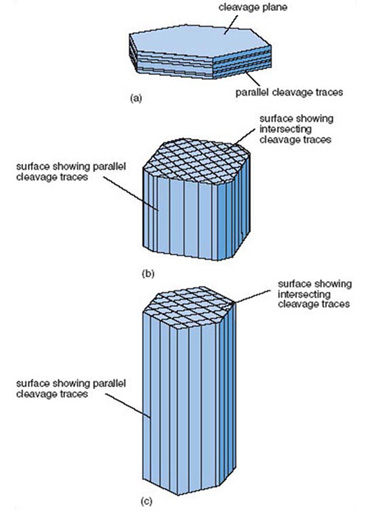
The most common silicate minerals found in igneous rocks (most of which are also common in sedimentary and metamorphic rocks) are listed in Table 1: minerals rich in silicon appear towards the top, and those poorer in silicon but richer in magnesium and iron appear towards the bottom of Table 1.
| Mineral | Diagnostic features | Chemical composition |
|---|---|---|
| Quartz | Colourless to pale; glassy lustre (broken surfaces similar to broken glass); curved fractures; no visible internal structure | SiO2 |
| Feldspar | Pale pink or white; two good cleavages | Ca, Na, Al silicates. Feldspar that is rich in Ca and Na is known as plagioclase, if rich in K and Na, alkali feldspar. |
| Mica | Forms platy crystals that split into flakes; one perfect cleavage. Brown or black mica is called biotite; colourless or silvery brown mica is called muscovite | K, Mg, Fe, Al silicate containing OH. Biotite is darker than muscovite because itcontains more Mg and Fe. |
| Amphibole | Dark green or black; elongate crystals with two cleavages (often hard to see) | Na, Ca, Mg, Fe, Al silicate with hydroxylgroup |
| Pyroxene | Dark green, dark brown or almost black; two good cleavages | Fe, Mg, Ca silicate |
| Olivine | Grass green; no cleavage, but has irregular fractures | Mg, Fe silicate |
Activity 2
Would you expect an igneous rock that contained a lot of magnesium (Mg) and iron (Fe), but not much silicon (Si), to contain all of the minerals in Table 1 in equal abundance?
Answer
No. The type and proportions of the minerals present must reflect the chemical composition of the rock. So an igneous rock that contains a relatively high proportion of Mg and Fe and a low amount of Si would be expected to contain more olivine and pyroxene and very little, if any, quartz.
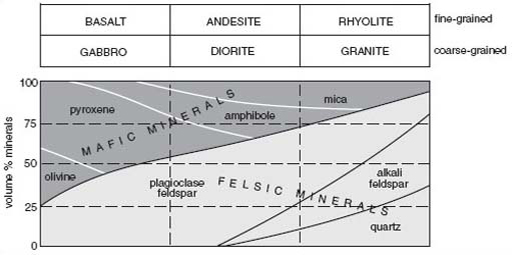
The variation in the mineral content of igneous rocks is shown in Figure 6, and this diagram provides the means to classify igneous rocks. The percentage of each mineral present is represented on the vertical scale, and the range of rock type (effectively the chemical composition) is given on the horizontal scale. Coarsegrained rocks (typical grain size greater than 2 mm) are named separately from finegrained rocks (typical grain size less than 0.25 mm). Notice that the proportion of pale-coloured minerals (felsic minerals) increases from left to right, reflecting the increase in the concentration of silicon in the chemical composition of the rocks. According to the diagram, granite is defined as a coarse-grained igneous rock containing quartz, feldspar (both alkali and plagioclase feldspar), mica and sometimes amphibole. The relative proportions of these different minerals are given by the width of the appropriate band on the diagram. For instance the quartz content of granite can range from about 10% to 35%, and the mica content from about 5% to 15%.
Question 1
Do igneous rocks with small crystals form at the surface of the Earth?
Give reasons for your answer.
Answer
Magma, such as volcanic lavas, that erupt at the Earth's surface will cool quickly so the crystals that form will be small, forming a fine-grained igneous rock.
On the other hand, magmas that are intruded deep in the Earth's crust will cool slowly giving large crystals time to form.
Question 2
Use Figure 6 to decide which of the following statements are true.
Olivine is not present in granite.
The proportion of pyroxene in gabbro can vary from about 25% to 50%.
Alkali feldspar is less abundant than plagioclase feldspar in coarse-grained igneous rocks.
A coarse-grained igneous rock containing 60% plagioclase, 15% pyroxene, 15% amphibole and 10% mica is classified as diorite.
A coarse-grained igneous rock containing 50% plagioclase, 35% pyroxene and 15% amphibole is classified as gabbro.
Amphibole can be present in gabbro, diorite and granite.
Answer
Statement 3 is false whereas all the others are true.
