2.5 Heat and life
The weight of evidence in the case of Europa points strongly towards ice overlying salty water, at least within the past few millions years although not necessarily today. There are signs that localised heating episodes have melted and fractured the ice. The intensity of tidal heating has probably waxed and waned in step with fluctuations in the amount of forced eccentricity of Europa's orbit, but we can anticipate that conditions on Europa would have varied through a broadly similar range during much of the Solar System's lifetime. What, then, are the prospects for life on Europa?
Let's consider the surface ice first. On Earth, active microbial communities have been found within Antarctic sea ice at temperatures as low as −18 °C. Here, algae and other organisms survive by photosynthesis in summer that is possibly supplemented when there is less light available by metabolising dissolved organic matter, but these are probably survivor species that need liquid water for part of their life cycles.
Question 18
Can you suggest four reasons why Europan surface ice is unlikely to be so hospitable for life as Antarctic sea ice?
Answer
Firstly, Europa's surface temperature of −140 °C even at the equator is far lower than in Antarctic sea ice, and we know of no way for water-based metabolism to proceed in such cold conditions. Secondly, liquid water would occur here far less frequently than within the Antarctic ice shelf. Thirdly, Jupiter is 5.2 AU from the Sun, so (according to the inverse-square law) the sunlight available for photosynthesis on Europa is some 27 times weaker than on Earth. Fourthly, unless there is a thriving ecosystem elsewhere on Europa, there would be no dissolved organic matter food source to supplement the energy available from photosynthesis.
Thanks to the escape of tidal heat, the temperature within Europa's ice is likely to increase with depth. However, even on Earth the light intensity is too low for photosynthesis to continue more than about 20 m deep within the ice. This is only a tiny fraction of Europa's ice thickness. There could be no ice warmer than −20 °C at a shallow enough depth for photosynthesis, except within very young matrix ice of chaos regions, or in the walls of fissures for brief periods during fracturing or ridge building eruptions. It is faintly conceivable that primitive photosynthetic organisms may lie entombed and dormant within Europa's near-surface ice for periods of millions of years, and become active only during relatively brief episodes of local heating (full-blown chaos generation, or above warm dome-forming intrusions as in Figure 23 in Section 2.3.4, or within an active fissure). This would be a pretty marginal existence. It is perhaps to the energy and nutrients that could be provided by hydrothermal vents that we must appeal if we wish to find the basis of a robust and persistent ecology of the kind imagined by Arthur C. Clarke (see Section 1.4).
Whether hydrothermal vents exist on Europa, and, if so, their abundance and their power, depend upon how deep within Europa the tidal heating occurs. This has not been determined, because it depends on unknown factors such as the strength and other properties of Europa's ice and rocky interior. At one extreme, virtually all the tidal energy could be dissipated within the icy shell (in which case chaos formation would be a result of direct heating of the ice). This would mean that the ocean was kept warm largely because of heat from above. Any hydrothermal vents on the ocean floor would be scarce and weak, and powered only by the feeble leakage of radiogenic heat from Europa's rocky interior. On the other hand, if tidal heating were concentrated in Europa's rocky part, flow of heat from the rock into the overlying ocean would be much stronger. As on Earth, ocean water would soak into the underlying hot rock, where it would become heated and react chemically, eventually escaping back into the ocean via hydrothermal vents. A static rocky substrate would not be very favourable for sustaining life because the ocean would deplete the available chemicals over million-year timescales. However, if tidal heating were sufficient to cause partial melting within Europa's rock, hydrothermal circulation would be especially strong over sites where igneous rock was being intruded at shallow depth, and strongest of all at any places where volcanic eruptions occurred onto the ocean floor. Moreover, the repeated arrival of new igneous rock at or a little way below the ocean floor would mean that the chemistry was continually renewed, so that some of the circulating water would always find something with which to react.
Question 19
Can you suggest why the presence of hydrothermal vents on Europa could be particularly important for the origin of life on Europa?
Answer
Phylogenetic evidence, in particular the ribosomal RNA tree, suggests that thermophylic autotrophic microbes dependent on chemosynthesis are the last common ancestor for life on Earth. Therefore life on Earth may well have begun at hot vents. If it did, then it could perhaps have begun with equal ease at hot vents on Europa.
An ocean of global extent would not have been necessary for life to begin. Relatively small pools of water sandwiched between ice and hot rock would have been enough. However an ocean, or at least an extensive body of water, would certainly make it easier for life to survive. Life that was trapped in a single pocket of water would have no escape when the hydrothermal vent that had been feeding it cooled down and ceased to flow. It would have to survive in a frozen state until the unlikely eventuality of a new vent starting up nearby. However, an ocean, or at least an extensive seaway, would mean that organisms (including free-floating larval stages of any multicellular life) could drift from vent to vent, allowing species to survive - even though individual colonies would meet their demise with the extinction of their vent.
The primary producers at hot-vent ecosystems on Earth derive their energy from a redox (oxidation-reduction) reaction. Typically, they exploit a reaction whose equilibrium position depends on temperature. For example if a high temperature (such as where hot fluids react with rock during hydrothermal circulation) drives the reaction in one direction but a low temperature (where vent water mixes back with ocean water) tends to drive the reaction the other way, then an organism can extract energy by getting involved in this 'reverse' reaction. This is only effective when the low-temperature ('reverse') reaction is kinetically inhibited, which provides the opportunity for a biological catalyst to become involved. (A chemical reaction is 'kinetically inhibited' when there is a significant energy barrier to be overcome to enable the reaction to proceed.)
An example of this in ocean-floor hydrothermal systems on Earth is the biological production of methane ('methanogenesis'). During hydrothermal alteration of newly created oceanic crust iron reacts with water. The iron is oxidised and the water reduced to hydrogen. Carbon is discharged in vent fluids as carbon dioxide, arising partly from oxidation of crustal and mantle carbon and partly from breakdown of carbonate rocks that have been drawn into the mantle at subduction zones. Thus, hot vent fluids are rich in carbon dioxide and hydrogen. In solution, these gases are related by the equilibrium reaction:
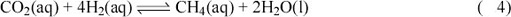
Note: when a chemical reaction is written this way, (aq) signifies something in aqueous solution, (l) signifies a liquid, (s) signifies a solid and (g) is a gas.
At high temperatures the equilibrium lies well to the left, so that in a hot solution carbon dioxide and hydrogen are stable. At lower temperatures, including those in seawater, the equilibrium position lies well to the right, but in a lifeless ocean an energy barrier would inhibit the reaction from moving in this direction. However, with biological mediation most of the carbon dioxide and hydrogen can react to form methane and water as the temperature falls. This is the reaction that methanogenic bacteria exploit as their source of energy.
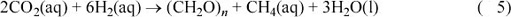
Note: (CH2O)n indicates carbohydrate in biological cell material, and the subscript n indicates that the real formula is more complicated than simply CH2O.
In principle, this reaction could be used by Europan equivalents of methanogenic bacteria at hot vents. There are reasons, however, why this particular reaction may not be a viable source of biological energy on Europa. One reason is that without (so far as we know) subduction of oxidised species, Europa's hydrothermal fluids are likely to be considerably more reducing than the Earth's. This would lead to vent fluids being naturally rich in methane rather than carbon dioxide, which would therefore deprive methanogens of their energy source. Another reason is that high pressure drives the reaction in Equation 4 towards the right. Now do Question 11, which compares the pressure on the Earth's ocean floor with that at the floor of Europa's ocean.
Question 20
The pressure on an ocean floor is given by the expression P = ρgd, which you have already met in a slightly different context in Box 7, as Equation 2 (see Section 2.4). For our current purpose, ρ is the average density of the overlying ocean, g is the acceleration due to gravity on the planetary body concerned, and d is the depth of the ocean. On Earth, we can take ρ to be 1030 kg m−3, g to be 9.8 m s−2, and d to be 3.0 km (the approximate depth of a mid-ocean ridge). On Europa, treating the ice thickness as negligible relative to the ocean thickness, we can take ρ to be 1180 kg m−3, g to be 1.3 m s−2, and d to be 100 km. Use these values to calculate the pressure at the exit of a hydrothermal vent on:
(a) the Earth's ocean floor
(b) Europa's ocean floor.
Answer
(a) Inserting the relevant values into Equation 2 (and remembering to convert from km to m), we get:
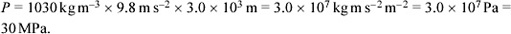
(kg m s−2 m−2 is force per unit area, which is pressure. The SI unit of pressure is the pascal, abbreviated Pa. Note that kg m s−2 m−2 could be written as kg m−1 s−2 but this would obscure the significance of kg m s−2 being the SI unit of force.)
(b) Similarly, inserting the relevant values into Equation 2 we get:
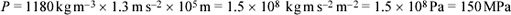
(or 200 MPa if we treat the 100 km depth to be valid to only one significant figure).
Thus, the pressure on Europa's ocean floor is about five times that at a mid-ocean ridge hydrothermal vent on Earth. This may not seem a big difference, and would be unlikely to have any adverse effect on, say, biological cell structure. However, it would affect the equilibrium in Equation 4, so that carbon tended to be outgassed as methane rather than carbon dioxide. The situation would be even less favourable for methanogenic life if Europa's subduction-deprived mantle is more reduced than the Earth's, because this would make the methane to carbon dioxide ratio very high in the first place. It would also mean that Europa is unlikely to provide favourable habitats for analogues of terrestrial SLiME (subsurface lithautotrophic microbial ecosystems).
Perhaps, then, biological methanogenesis is not viable on Europa. In an extreme case, Europa's hydrothermal fluids could be so reducing that the only plausible oxidants that could provide an energy source for life would be oxidised metals, such as ferric iron (Fe3+). A suitable reaction is represented by:
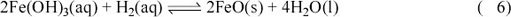
in which the iron in vent fluids is reduced by reaction with hydrogen. Alternative reducing agents could be hydrogen sulfide or even methane. In all these cases, biological organisms could feed off the energy released during reduction of Fe3+ to Fe2+.
On the other hand, it is conceivable that Europa's ocean may actually be moderately oxidising in character.
Question 21
Can you recall from earlier in this course a mechanism whereby molecular oxygen is known to be generated on Europa?
Answer
In Section 2.2 you learned how exposure to charged particles and solar ultraviolet radiation in the near-surface ice leads to radiolytic and photolytic breakdown of water molecules to produce oxygen and hydrogen.
The hydrogen escapes relatively easily to space, but much of the oxygen is held within the ice crystals. These processes are only effective in the upper few micrometres (μm) of the ice, but 'gardening' by micrometeorites and slightly larger impacts can be expected to mix the products to a depth of about 1 m in the regolith. We do not know how efficiently, if at all, such oxygen is eventually mixed into the ocean, but obviously this could occur from time to time when melting, especially during chaos formation, reaches the surface.
There is actually a radiolytic mechanism whereby oxygen could be generated from either ice or liquid water at any depth below the surface. This is because one of the common elements thought to be dissolved in the Europan ocean has a radioactive isotope.
Question 22
Look back at Figure 15 (see Section 2.2), and see if you can recognise which of these elements has a radioactive isotope.
Answer
The element with a radioactive isotope is potassium.
The radioactive isotope is 40K, which on Earth, and presumably Europa too, makes up about 0.012 per cent of the total potassium today, and would have been about ten times more common shortly after Europa was formed. β-particles and γ-radiation are emitted by 40K as it decays and both can radiolytically break water into hydrogen and oxygen, by means of the series of reactions indicated in Box 4 (see Section 2.2).
This process could yield about 1010 moles of oxygen per year in Europa's ocean. Provided there is sufficient carbon available and a suitable reaction pathway, this would be enough to support about 107-109 kg yr−1 of biomass production. However, the limited availability of carbon in the right form and right place almost certainly means that the actual rate (if any) of biomass production in Europa's ocean is probably less than this. A likely value, allowing for a modest amount of hydrothermal energy, is about 105-106 kg yr−1.
Question 23
Rates of biomass production on the Earth today are about 5 ×1013 kg yr−1 by photosynthesis on land, a similar amount by marine photosynthesis (mainly by microscopic plankton), and about 1010 kg yr−1 by chemosynthesis at ocean-floor hydrothermal vents.
How do these rates of biomass production compare (by orders of magnitude) with the value proposed for Europa?
Answer
Europa's annual biomass production is estimated to be at least eight orders of magnitude less than that of present-day Earth (a maximum of 106 yr−1 on Europa versus a total of about 1014 yr−1 on Earth). Even if we compare only chemosynthetic biomass production, Europa is estimated to be at least ten thousand times (four orders of magnitude) less productive.
It is important to remember that the estimates for Europa are very uncertain, and could be underestimates by two or three orders of magnitude - or ridiculous overestimates if Europa supports no life at all. However, Europa could offer sites that are just as favourable for life to have originated as those on the early Earth, and equally hospitable for so-called extremophiles to flourish today, albeit in smaller quantities than on Earth. Europa's small mass (so small that it cannot hold onto an atmosphere) and its distance from the Sun beyond the 'habitable zone' have prevented it from developing a photosynthesis-dominated biosphere, but the viability of any chemosynthetically supported biosphere is independent of this. Thus, according to some assumptions at least, the prospects for life on Europa appear encouraging.
Whether any life has remained at the level of simple single-celled autotrophs or diversified into multicellular forms, and whether any heterotrophic organisms have evolved to prey on these (as imagined by Arthur C. Clarke) remains to be seen.
We will conclude our discussion of Europa with a brief look at plans to gather further data on this intriguing world.
