3.2 Lighting inventions
The Davy lamp is just one example of an inventive solution to a problem. In that case, existing materials were used to provide the answer. In some cases, though, the development or use of new materials is part of the inventive process, and the new material allows the creation of new products. In this section I will look at some lighting inventions that progressed through materials development.
Although flame safety lamps were only of limited applicability outside coal mines, there was a long-felt need for brighter lighting during much of the 19th century. With the growth in manufacturing industry, complex machines needed continuous supervision and maintenance, and illumination by candles or oil lamps was far from adequate (even during daylight hours). Although intense sources of light had been known since Faraday's work on The carbon-arc lamp , they were not readily portable and were not safe or cheap enough for regular use either in industry or in homes.
The carbon-arc lamp
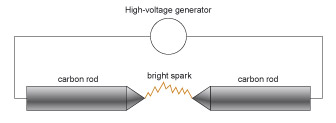
The carbon-arc lamp was one of the first light sources to exploit the discovery of electricity. The device was fairly simple: a very high voltage was generated and applied to two carbon rods. Holding these rods close together produces a spark between them (Figure 39) because a high electric field is produced in the air gap between the ends of the rods. With a powerful electricity source, the spark will be continuous, and can be extremely bright.
The problem with this design is that the rods are burnt off rapidly, and so need to be continuously pushed together in order to maintain the electric current and hence the light. If the gap between the rods becomes too large, the electric field will drop to a point where the spark will no longer form.
The development of coal gas – flammable gases extracted from coal – provided a readily available source of energy when piped distribution systems were constructed. However, the illumination from naked flames was not great, being not much better than a candle, and there was the continuing danger from fire. This problem did result in the invention of the incandescent mantle, where the flame is played on a woven structure impregnated with metal salts that shine brightly, i.e. are incandescent, when heated in an open flame. A similar development used lime as the incandescent material (hence the expression 'limelight').
One problem with all these methods is that a high temperature is generated by the light source, which could clearly be a fire hazard. There was no 'safe' lighting system available apart from miners lamps in collieries.
Although the concept of electricity had been understood for some time, the development of electricity-generating systems (pioneered by Edison in the USA) encouraged efforts to develop a light source powered by an electric current. If this could be done successfully, then electric light could be the solution to low intensity (candles, oil lamps) or dangerous (carbon-arc, gas flame) lighting. Although electric lighting works by heating a wire until it becomes hot enough to glow brightly (it becomes incandescent ), the invention of the light bulb neatly side-steps the danger of having exposed high temperatures by enclosing the incandescent source in a glass envelope. The key to the solution of the problem of electric lighting lay not so much in the need for an electric current, since this was already available, but rather in the need to find a material which could withstand:
- the high temperatures needed to reach incandescence and
- glow brightly without melting;
- which would conduct electricity, and
- which would last for a reasonable time.
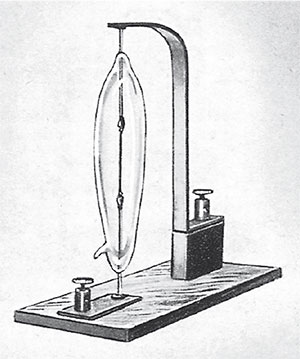
The solution was found independently by Edison in the USA and Swan in Britain in about 1879. Heated carbonised filaments would provide a continuous light if they were protected from oxidation (i.e. burning) by being enclosed in either an inert atmosphere (which does not react with the filament) or a vacuum (Figure 40). Both solutions worked, although the lifetime of the lamps was still limited by today's standards. Nowadays, the preferred filament material is tungsten. It possesses a very high melting point (3410 ºC) and can be readily fabricated into the coiled wire filament of electric light bulbs.
Activity 17 (self-assessment)
- a.What was the key problem in making the first incandescent electric light?
- b.What had been the previous methods used for producing light artificially? What were the perceived problems with these methods?
- c.Why was choice of material important for the electric light filament?
Answer
- a.The critical problem in making an incandescent electric lamp was finding a material that would resist burning in air at the high temperatures created when an electric current flowed through the filament.
- b.The previous practices (the 'prior art') in the field of illuminating lights before 1879 included:
- open flame lamps or candles
- flame lamps with a mantle
- lime lights
- carbon arcs.
Open flame lamps provided very little illumination, but it could be increased by providing a mantle around the flame. These were composed of a woven structure impregnated with chemicals (inorganic salts) which increase the illumination level. One such salt was lime, which created an intense light when heated by an open flame. The carbon arc lamp consisted of two electrodes supplied with electricity which, when close enough, allowed a continuous spark to travel between the electrodes. Although the arc provided a very bright light, the carbon rods burned away rapidly.
- c.The material choice for a filament for an electric lamp is important because the following properties are needed:
- very high melting point
- electrically conducting
- capable of being made into a fine filament.
There is much interest in improving the efficiency of the incandescent lightbulb. One innovation involves halogens such as bromine and iodine. Small amounts added to the atmosphere in the bulb increases the efficiency by allowing the filament to work at a higher temperature, and halogen bulbs are used in car lights as well as in domestic lights. Fluorescent bulbs have been promoted for their greater efficiency and lower working temperature, although more expensive to purchase. Light-emitting diodes (LEDs) have also been developed, being of simple construction and greater efficiency. Domestic light bulbs using LEDs are available, although at some great expense. They are also now widely used in small torches.