4.2.2 Properties and internal materials structures
So far I have principally treated materials just as 'stuff' that has a series of properties. You have seen that these properties vary from material to material but I have not really started to discuss why they do. I am not going to go into this in any real depth in this course but there is one important aspect of materials engineering that you have to know about before exploring materials processing much further. This is to do with the structure of materials across a whole series of size scales ranging from a few millimetres down to a few Ångströms (symbol Å; 1 Å = 10 –10 m or one ten-billionth of a meter!).
We are all familiar with the human scale of tangible products ranging from a cup all the way up to a building or a bridge. We call this scale macrostructure – 'macro' from the Greek word for 'large'. You should also be familiar with the concept that the properties of materials depend to a degree on their structure at the other extreme of scale – the type and arrangement of their individual atoms and molecules. This is usually called atomic (scale) structure. However, much of materials engineering is concerned with a size scale in between – generally too small to be seen with the naked eye, but much larger than individual atoms and molecules. This middle ground is termed microstructure – 'micro' from the Greek for 'small'.
The properties of solid materials can be profoundly influenced by their microstructure. And because a material's microstructure is almost invariably changed by the manner in which it is shaped into a product, the properties of materials in products are dependent on how they are processed.
You've already seen in earlier sections the importance of materials properties and the component geometry. Component geometry is an example of structure on a macroscopic scale. Look at Figure 58(a), which shows the second Severn Crossing. The bridge has the structure it does because it was built to achieve the task of providing a path for vehicles across the estuary, at an acceptable cost and with complete safety during construction and during use. The central portion is an example of a cable stayed bridge, where the deck of the bridge is attached to the supports by cables. This structure was chosen, presumably, as being the best solution, although there may have been several alternative structures that would have performed equally well with the final decision being made on aesthetic grounds.
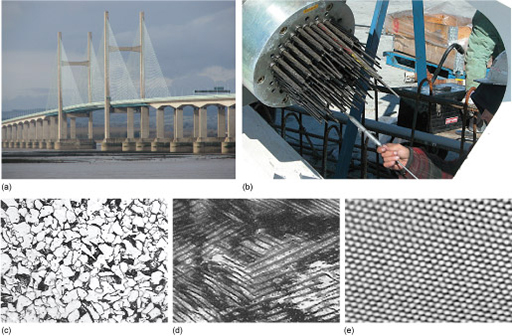
If we look at the structure of a support cable for such a bridge (Figure 58(b)), we see that it is not a solid bar of material, but is 'woven' from many thinner strands of wire. This structure (still a macrostructure) is chosen for several reasons, including safety. With a reasonable safety factor, it shouldn't matter if a flaw causes the failure of one wire strand because there are multiple paths for the load that the cable is supporting. In addition there are some beneficial properties that cable structures have compared to large single strands, such as flexibility.
The structure story doesn't stop with the material for one strand, though. I've already indicated that steel is a mixture of iron with carbon, and the way carbon affects the structure of the iron on a microscopic scale depends on the amount of carbon in the iron and the heat treatment that the iron has had. Figure 58(c) shows the microstructure of a typical steel. This shows that as we look in closer detail we begin to see that what we thought was quite a smooth, plain metal surface has a lot of underlying structure to it. Once we've zoomed in so that we can see features as small as 10 micrometres, it becomes clear that the metal is composed of small individual 'grains'. This structure in turn determines the mechanical properties, like strength and toughness, of the steel. We can control the microstructure of the iron: through alloying and heat treatment the grain size and structure can be altered, so tailoring the properties of the material that we make.
Figure 58(d) zooms in still further, showing us more of the structure within the grains themselves. Influencing things at this level is more complicated, but it can be done, and again can help to tailor the material properties.
Finally, we can zoom down to the level of the atomic structure (Figure 58(e)). In this case, we're looking at carbon, one of the elements in steel. The bonding between the atoms, and the structure they take up, critically influences the material properties, but there's nothing we can do to change it!
Some materials are more useful than others because they have the right sort of atomic bonding and atomic structure, and a microstructure that we can do useful things with. In Figure 58(e), you can see that each carbon atom is surrounded by six others in an hexagonal pattern. This is simply the way that carbon atoms arrange themselves in this instance (carbon is versatile in that it can adopt several atomic arrangements).
We will refer to microstructure frequently in this section. It is a key factor in determining mechanical properties, and it can be greatly affected by the choice of manufacturing process for a material.
