5.2 Simple electrochemical cells: invention or discovery?
Before Galvani reported his work on animal electricity in 1791, Volta had been preoccupied with the static electrification of substances, deriving his electric charges from things being rubbed. The various machines available could do the work necessary to separate the electrical charges involved by a considerable distance, investing in them a large amount of energy. However, this energy was soon spent in brief, but spectacular, sparks.
The pulling apart of electrical charges that the machines achieved was rather like the stretching of elastic bands: strong forces build up, directed towards restoring the initial arrangement.
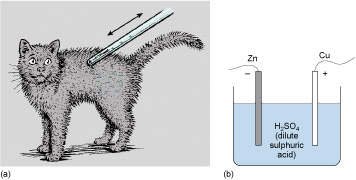
By contrast, Volta found that the electricity created by the contact of dissimilar metals through a chemical solution (i.e. chemical energy) appeared to be of much lower energy. It was found that although displays of sparks could be made, they were feeble. Importantly, though, these sparks persisted for as long as the metals were joined through the various chemical solutions, particularly acidic ones (see Acids and alkalis ); see Figure 81. Today we call an arrangement in which two metals are joined through a chemical solution an electrochemical cell, because what happens in the cell involves both electricity and chemistry. An electrochemical cell is the basis of all batteries.
Acids and alkalis
You will probably be familiar with the word 'acidic'. An acid is a chemical solution that has a high concentration of hydrogen ions (a hydrogen atom minus its electron). An alkali, which may be considered to be the 'opposite' of an acid, has a low concentration of hydrogen ions. Here 'high' and 'low' are generally taken to be relative to pure water. Water, formula H 2 O, does not have all its constituent atoms joined together as the formula suggests. In practice, some of the H 2 O molecules will split apart in the water to form positively charged H + (hydrogen) ions, and negatively charged OH – (called 'hydroxide') ions.
A solution that has a higher concentration of H + ions is an acid; one with a higher concentration of OH – ions (which will tend to mop up stray H + ions and so reduce their concentration) is an alkali. You won't have to remember these facts, but it's useful to know the definitions. A measure of the concentration of hydrogen ions – and so a measure of the acidity – is the pH number. You may have seen claims that shampoos are 'pH balanced with your hair'; or if you are a serious gardener, you may have had cause to check or adjust the pH of your garden soil.
Still curious, Volta discovered that not all metal combinations 'tasted' the same – their 'effectiveness' (the apparent strength of the interaction) differed. This may sound like a rather subjective approach to scientific research, but it must be remembered that the electric instruments we use today have all been developed following Volta's work.
Volta published a list of metals in an order that reflected their efficacy in terms of strong taste:
zinc, tin, lead, iron, copper, platinum, gold, silver, graphite.
The further apart any pair were in this list, the greater the stimulation of the taste buds. You may have experienced this effect yourself if you have traditional amalgam fillings in your teeth (based on mercury); a scrap of aluminium foil from a chocolate-wrapper or a metal fork touched directly against the filling will give the tongue a bitter-tasting twinge.
The inclusion of carbon (graphite) in Volta's list is interesting. As a conductor of electricity, graphite was apparently to be considered a metal.
Activity 38 (self-assessment)
Which pair from Volta's list is likely to give the greatest sensation on the tongue?
Answer
Zinc and graphite, being furthest apart, are likely to produce the greatest effect.
A more convenient way to assess different combinations of materials was using electrochemical cells like the one shown in Figure 81(b). Volta was able to investigate this chemical electricity using techniques he had devised for frictional electricity. In light of these experiments, Volta modified his ordering of the electrochemical effects of dissimilar metals. His final order reversed the positions of tin and lead and those of silver and gold.
Activity 39 (self-assessment)
Did Volta invent or discover the electrochemical cell?
Answer
It looks like discovery to me. There was no real application for such a cell at that stage.
There was a long and controversial debate among European scientists as to whether the Galvani observations were manifestations of animal electricity or were entirely accounted for by Volta's description of the behaviour of dissimilar metals. As we now know, both were right to some degree. As modern electrocardiographs – like those used to monitor heart activity in hospitals – show quite clearly, animals are electrically active. Indeed, the nerves themselves communicate by peculiar electrical means.
