Ionic radii of the lanthanides
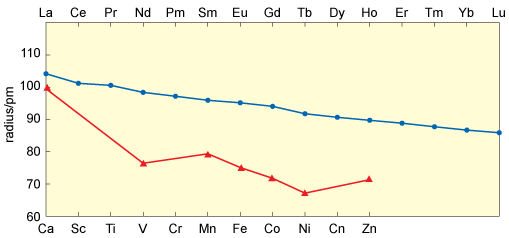
If you now consider the ionic radii of the lanthanide ions (Figure 11), they show a smooth decrease across the series.
This is often referred to as the lanthanide contraction.
In essence, this reduction in size of the lanthanide ions results from poor shielding of the positive nuclear charge by the 4f electrons which pulls the 6s electrons towards the nucleus. This has important consequences for the Periodic Table, as the size of the last four ions – and – falls below that of in the preceding transition series. This means that the elements that follow yttrium in the third transition series are much smaller than expected.
But if you make a comparison with the ionic radii of dipositive ions of the first transition series (Figure 11) where a double-bowl variation is seen. This reveals an important feature of the coordination chemistry of the lanthanides.
-
How is the double-bowl shape of the first transition series explained?
-
This arises from the influence of crystal-field effects superimposed on the effect of increasing nuclear charge.
-
What does the smooth lanthanide contraction tell you about crystal-field effects in lanthanide compounds?
-
They are much smaller than in first-row transition metal compounds, which supports the notion that, in lanthanide compounds, the 4f orbitals are almost part of the core.