Paramagnetism of lanthanide complexes
When considering the lanthanides in the context of MRI contrast agents, the magnetic properties of their complex ions are important.
There are two possible contributions to the paramagnetism of a transition-metal complex. One arises from the spin of the unpaired electrons.
-
From what does the other contribution arise?
-
The orbital angular momentum of the unpaired electrons.
You know that for transition metal complexes, the d orbitals are strongly split by the crystal field.
This splitting can quench the orbital angular momentum meaning that for first-row transition metal complexes, the paramagnetism arises almost entirely from the spin of the unpaired electrons.
The magnetic moment is close to the ‘spin-only’ value and Equation 1 can be used to determine its magnitude.
Recall that μS is the spin-only magnetic moment, n is the number of unpaired electrons, and μB is the Bohr magneton.
But for lanthanide complexes this isn’t the case – take a look at Table 3.
| Ion | Electronic configuration | μ/μB |
|---|---|---|
| La3+ | 4f0 | diamagnetic |
| Ce3+ | 4f1 | 2.51 |
| Pr3+ | 4f2 | 3.53 |
| Nd3+ | 4f3 | 3.55 |
| Pm3+ | 4f4 | 2.68 |
| Sm3+ | 4f5 | 1.46 |
| Eu3+ | 4f6 | 3.37 |
| Gd3+ | 4f7 | 8.00 |
| Tb3+ | 4f8 | 9.33 |
| Dy3+ | 4f9 | 10.55 |
| Ho3+ | 4f10 | 10.40 |
| Er3+ | 4f11 | 9.50 |
| Tm3+ | 4f12 | 7.35 |
| Yb3+ | 4f13 | 4.30 |
| Lu3+ | 4f14 | diamagnetic |
The magnetic moments are plotted in Figure 12, along with the spin-only values calculated from Equation 1.
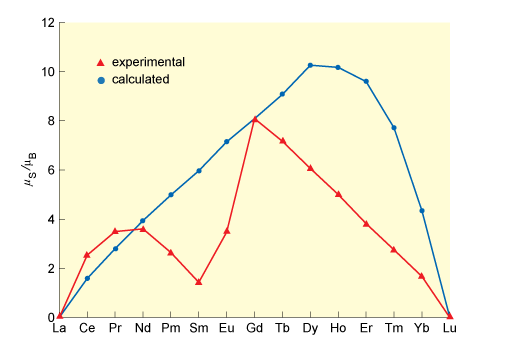
The clear failure of the spin-only formula shows that the orbital angular momentum is not quenched in the way that it is in first-row transition-metal complexes.
-
What does this suggest about the splitting of the 4f orbitals in lanthanide compounds?
-
It must be small (backing up what you saw in the previous section), and not sufficient to quench the orbital angular momentum
This, in turn, suggests that the exposure of the 4f orbitals to the ligands is small, and is further evidence that the 4f electrons are close to being part of the noble-gas core.
The magnetic moments in Table 3 are very similar to those in other lanthanide compounds, and are characteristic of the configurations set alongside them. They can therefore be used to identify the configuration concerned.
Note that any 4f configuration is associated with just one high-spin magnetic moment.
-
How does this differ from complexes of the d-block metals?
-
Certain d-electron configurations occur in both high- and low-spin states.
-
Why is this further evidence that crystal-field effects are small in lanthanide compounds?
-
Low-spin complexes would require a large crystal-field splitting in the 4f orbital energy levels.
You will now be looking in detail at MRI contrast agents and, as you’ll see, the magnetic properties of the lanthanide ion play a key role.