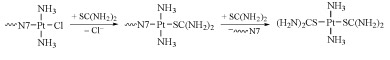5.5 Why is transplatin inactive?
As you saw previously, transplatin is not active. The distance between the two chlorine leaving groups is longer than in cisplatin (Figure 24), resulting in transplatin being unable to form 1,2-intrastrand DNA adducts similar to cisplatin.
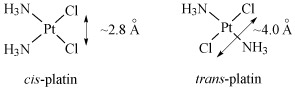
It can, however, form 1,3-intrastrand (G–p–N–p–G), 1,4-intrastrand and 1,4-interstrand links as well as monoadducts.
The lack of anticancer activity with transplatin is believed to be because transplatin lesions are more easily repaired than those of cisplatin, as they lead to more radical distortion of DNA. They don’t bind HMG proteins as strongly as cisplatin lesions, possibly because of the lack of an appropriate space for insertion of the HMG protein phenyl group in the manner described above.
In addition, transplatin is more readily intercepted by sulfur-containing species (e.g. glutathione, GSH, Structure 13), leading to the removal of platinum from the cancer cells (Equation 3).
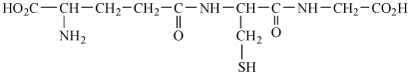
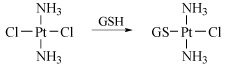
In addition, monoadducts of transplatin are displaced by the action of trans-labilising nucleophiles such as glutathione or thiourea (Equation 4).