2.11 Carbon
The following video looks at a carbon atom, exploring its size and inclusion in diverse organic molecules. Carbon is ‘fixed’ by plants through photosynthesis – by which you mean incorporated into molecules used by living organisms. Here you can explore the length of time the carbon is stored in various forms after a plant dies before being released into the atmosphere again as CO2.
Humans use fossil fuels to power many of the things used in your daily lives. These fossils fuels are energy stored in carbon molecules fixed through photosynthesis that occurred hundreds of millions of years ago, during the Carboniferous and Jurassic periods.
While watching the video, record the following information to use in the questions that follow:
- the diameter of a carbon atom
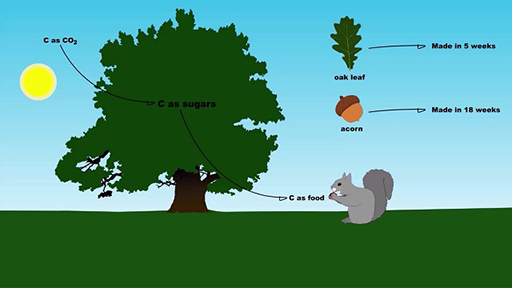
- how long it takes an oak leaf and an acorn to grow
- the dates of the Carboniferous and Jurassic periods, when much of the carbon forming today’s coal, oil and gas reserves was fixed though photosynthesis.
Transcript: Carbon in energy sources
Question 1
What is the diameter of a carbon atom?
Question 2
The video explained that you cannot see an individual carbon atom because they are shorter than the wavelengths of visible light. The shortest wavelength of light that you can see is about 390 nm (which is violet light). How many orders of magnitude longer is this than a carbon atom?
Question 3
Carbon is part of glucose molecules. Choose the appropriate words below to compare the sizes of a single carbon atom to a glucose molecule.
Many of our fuel sources use carbon-based compounds as sources of energy, from foods that you metabolise to wood, coal, crude oil and natural gas that you burn. Different sources vary in the amounts of energy released per unit mass.
Table 8 lists different fuel sources, the amount of energy released per kg, and the amount of CO2 emissions for the energy produced. Joules (J) is an SI unit that describes energy; you will have seen it listed on food wrappers.
| Energy source | Net calorific value/MJ kg−1 | CO2 emissions/g MJ−1 | % Carbon |
|---|---|---|---|
| Wood and leaves | 16 | 4 | 42 |
| Coal | 29 | 115 | 67 |
| Natural gas | 38 | 50 | 76 |
| Crude oil | 42 | 72 | 89 |
Question 4
Use information from the video to determine the age of the different energy sources in Table 8. (Note that for coal, natural gas and crude oil you should express your answer using scientific notation.)
Question 5
Tabulated information, such as Table 8, facilitates comparisons between values. Select the correct words in the sentences below to describe the patterns in energy and carbon content and emissions of the different energy sources.
Discussion
The amount of CO2 emissions depends on the carbon content and the ratio of carbon: hydrogen in fuel. Although all energy sources are formed from the fixation of carbon dioxide through photosynthesis, wood, coal and gas are made of different compounds. Gas, often methane (CH4), has a higher ratio of hydrogen to carbon in comparison to coal and wood, and therefore lower CO2 emissions than coal for a given energy release.
Question 6
Returning to the oak tree, how long does it take for an oak to produce an oak leaf? Use the equation below to aid your conversion from weeks to seconds. You can identify pairs of units that occur both in the numerator and denominator, which will cancel each other and give the final units for the answer. (The cancelling of weeks are shown as an example.) Leave no spaces between digits in your answer.
Discussion
Note that this is a special case where each number is an exact integer. Each day has exactly 24 hours, each minute has exactly 60 seconds, etc, so you can report the answer as 3024 000 seconds.
Question 7
Organisms including oaks use carbon to make many different types of molecules. Acorns are full of carbon-based sugars and starches to fuel the embryo when an acorn germinates; hence acorns are an attractive energy source for animals.
Oaks also use carbon to make defensive compounds to deter animals. The English oak makes compounds called sesquiterpenes which have been shown to deter insects. One of these compounds, farnesene, contains 15 carbon atoms of which 13 form a long carbon chain. Hence the molecule has a length of roughly 1.8 × 10−9 m (calculated as 13 × 1.4 × 10−10 m).
Use this size and the time it takes an acorn to grow to add the bar ‘Acorn and sesquiterpenes production’ to the size–time explorer [Tip: hold Ctrl and click a link to open it in a new tab. (Hide tip)] . You can use the same calculation as you used in Question 6 to calculate the length of time it takes for the oak to produce an acorn.
Summary
After learning about carbon atoms and how small they are, you learned that carbon is a component of many of our fuel sources. The food you eat is produced relatively quickly – as is the wood you burn – but fossil fuels are ancient sources of carbon that were fixed by plants many millions of years ago. They cannot be replaced quickly as it takes time to build up large reserves of these compounds, of which tiny carbon atoms are a significant component.
Next: Now look at even smaller scales by examining the components of an atom, which you can do with the ‘Next >’ button, or you can return to the size–time explorer to choose a level for yourself.
