2.5 The uncertain Universe
Despite the impact of relativity, the greatest source of change in the scientific world-view in the twentieth century has undoubtedly been the development of quantum physics. This is the branch of physics that is mainly concerned with microscopic entities such as atoms and molecules, and their constituents. It is by far the most quantitatively accurate part of science, routinely providing predictions that are correct to just a few parts in a million. Quantum physics is also of enormous technological importance since it provides the scientific underpinning for the modern electronics industry, which brings us devices ranging from TV sets and transistor radios to CD players and computers.
So great has been its effect that it is now conventional to divide physics into two parts; quantum physics and classical physics, where, by classical physics, we mean anything that is not quantum physics. To be fair, it should be noted that some authors prefer to define classical physics as consisting of those subjects that were already well-defined by the year 1900, together with their direct developments in the twentieth century. In this way they include mechanics, thermodynamics and electromagnetism, but they exclude special and general relativity. Most physicists, however, would not hesitate to say that general relativity was a classical theory of gravity, and would regard relativity as the culmination of classical physics rather than a step beyond it. In any event, there can be no doubt that the development of quantum physics has demanded a fundamental change in outlook by physicists.
Quantum physics was born in 1900, but it took about 25 years to reach maturity. During the first quarter of the twentieth century it had a rather rickety feel; there was not really any coherent theory of quantum physics, just assorted quantum ideas that were so successful in solving certain outstanding puzzles that it seemed there had to be something behind it all. The strongest characteristic of quantum physics during this early period was an emphasis on graininess or discreteness.
Indeed, the word quantum actually comes from the Latin for 'unit of quantity' or 'amount' and was introduced into physics by the German scientist Max Planck (1858-1947), in the course of his investigations into the emission of electromagnetic radiation from hot surfaces.
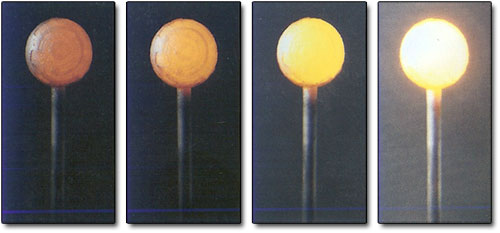
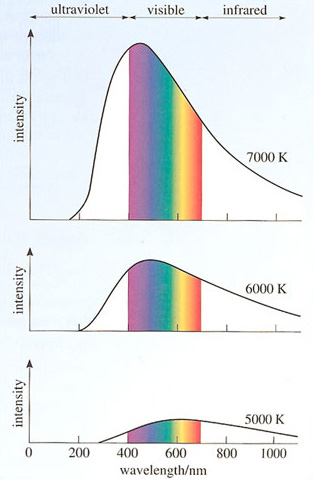
Crudely speaking. Planck was looking into why hot things glow. He knew that the light given off by a heated object is a mixture of all the possible colours of light and he wanted to predict the relative brightness with which each colour would be emitted from an object at a given temperature. It was changes in these relative brightnesses as temperature increased that explained why objects went from being red-hot at fairly low temperatures to white-hot or blue-hot at fairly high temperatures (Figure 30).
Planck found that, in order to account for the observed pattern of emission from hot bodies, he had to assume that energy was transferred from the heated surface to the emitted radiation in a 'grainy' way. Corresponding to each particular colour of light there was a minimum amount of energy - a quantum of energy - that could be carried away from the surface by the light. The size of this quantum of energy depended on the colour of the light; an energy quantum of violet light was almost twice as energetic as an energy quantum of red light, and every other colour had its own characteristic quantum. Planck was able to write down a law that related the quantum of energy corresponding to any particular colour to the physical property (frequency) which determined that colour. In doing so he introduced a new fundamental constant of Nature - now called Planck's constant (h = 6.626 × 10−34joule seconds). The appearance of Planck's constant in a calculation can be taken as a clear indication that quantum physics is involved.
Planck's law was used with great success over the following quarter of a century, in a variety of contexts. Einstein used it in his 1905 paper explaining the photoelectric effect, and so did the Danish physicist Niels Bohr (1885-1962), in 1913, when he formulated a theory of the inner workings of the atom that achieved some remarkable successes in spite of a number of unsatisfactory features. It showed up again in 1924 in the doctoral thesis of Louis de Broglie (1892-1987), who suggested that entities which are normally thought of as particles, such as electrons, actually have a wave-like aspect to their behaviour. Einstein, Bohr and de Broglie all received Nobel Prizes in recognition of their work.
These early developments were strikingly out of step with conventional classical physics. They might even be described as revolutionary, but the real revolution was still to come.
