2.1 Why is water so important?
To understand why water is important for life, we need to know about the structure and the chemistry of water.
The two hydrogen atoms are not exactly opposite each other, but are instead at an angle. Video 1 shows a three-dimensional representation of water molecules, with the atoms in a water molecule (two hydrogen atoms and one oxygen atom) represented by spheres. Watch this video now.
Transcript: Video 1 three-dimensional representation of water molecules.
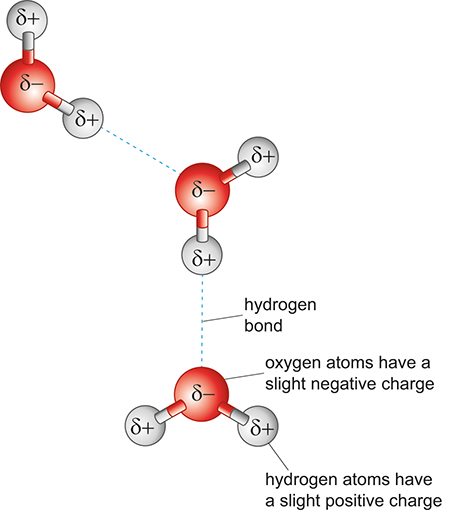
The bonds that join the oxygen and hydrogen atoms in a water molecule are made up of electrons shared between the atoms. However, the electrons are more strongly attracted to the oxygen atom than to the hydrogen atoms. This means that, near to the oxygen atom the molecule has a slight negative charge, but near the hydrogen atom the molecule has a slight positive charge. We call molecules with this feature polar and it is this polarity that makes water special. For example, it can form large networks of water molecules where the positive charge of a hydrogen atom attracts the negative charge of an oxygen atom - this forms a hydrogen bond between water molecules. This is shown on Figure 8, which is a different method of visually representing molecules – a ball and stick model – in which the atoms are shown as spheres and the bonds between them as sticks.
The three-dimensional shape of hydrogen bonds, and the polarity of water molecules is the main reason for some of the unusual properties of liquid water, such as its high surface tension and the fact that ice floats on water. It is also why water is an extremely good solvent for solids, liquids and gases. These properties have critical roles to play in why water is a key target in the exploration of Mars.