7 Electric vehicles
Battery electric vehicles
The concept of third-generation biofuels is to cut or eliminate pollutants in the fuel production process. This fuel-switch approach also includes policies to promote electric and hydrogen vehicles, which enable pollutants to be reduced either during manufacture (e.g. in generating electricity at power stations/hydrogen at refineries) or by using renewable sources of energy that produce little pollution at all. The move towards such a fuel-shift strategy thus brings together action to cut transport's local and global environmental impacts. It is also one that has the potential to link to the powerful political driver of energy security.
Although the use of batteries and electric drive in hybrid cars is now mainstream, an entirely electrically powered car requires the storage of large amounts of energy on board the vehicle. One way to do this is, of course, in batteries. Older types of battery electric vehicle (BEV) used lead acid batteries, but most current electric vehicle designs use lithium-ion (Li-Ion) and lithium-polymer (Li-Poly) traction batteries. These have a much higher energy density (100–125 W per kg), providing a significant improvement in driving performance and vehicle range.
First-generation electric vehicles used direct current (dc) motors, but more recent cars convert the direct current to alternating current (ac) using an inverter, which then drives an induction motor. These vehicles have increased efficiency, have a higher specific power (per kg) and require less maintenance.
Until recently, BEVs were only available in small numbers as variants of ICE cars (e.g. the Peugeot 106 electric car, manufactured from 1995–2003). A dedicated BEV design, the REVA G-Wiz micro car (legally classed as a 'quadricycle'), was launched in 2001 and has secured a small niche market, selling 4000 vehicles worldwide by 2011. Renault's recently launched 'Twizzy' is also an electric 'quadricycle'.
More significantly, since 2010 a number of dedicated high-performance BEVs have been launched commercially, including the Mitsubishi iMiEV, the electric Smart, Nissan's Leaf (Figure 14), the Peugeot iOn, Renault's Fluence and the Teslar Roadstar 210 kph sports car. The Mitsubishi iMiEV EV technology [Tip: hold Ctrl and click a link to open it in a new tab. (Hide tip)] website provides a good overview of key features of modern BEVs; if you wish, you can also follow this link to find BEVs available in the UK.

One of the main concerns about BEVs is that they only have about a 160 km (100 mile) range and that in most cases recharging is slow (6–8 hours). They also cost about a third more than a comparable ICE car, although much-reduced running costs counteract the high initial purchase price.
Promoting battery electric vehicles
The commercial launch of a range of BEV designs is part of a UK government/industry partnership approach that envisages a long-term transition to a low-carbon transport future in which cleaner internal combustion technologies are joined by an initial widespread uptake of battery electric vehicles and then 'plug-in' hybrids, followed later by hydrogen fuel cell vehicles (New Automotive Innovation and Growth Team (NAIGT), 2009). Similar programmes have taken place in France, Germany, Spain and the USA. Figure 15 shows the technology uptake 'roadmap' from the 2009 NAIGT report.
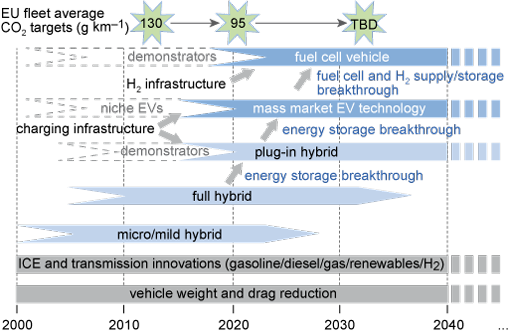
Note that this roadmap envisages that BEVs will only initially have a niche market, which will pave the way to mass-market EVs from 2020 onwards. The roadmap includes EU fleet average targets for CO2 emissions, but does not indicate a target beyond 2020.
This long-term strategy is supported by government programmes to encourage the uptake of BEVs. These have included, up to 2015, purchase subsidies (worth up to £5000) to help overcome the high initial cost of battery electric cars, and a programme to provide recharging infrastructure through grants to local authorities. The full UK programme is coordinated by the Office for Low Emission Vehicles. (For further information, see 'Plug-in Car Grant' and 'Recharging infrastructure' in Department for Transport, 2012b).
Activity 9 (exploratory)
BEVs are a good illustration of the different cost structure of low-carbon vehicles. Table A.3 shows some key costs of the Nissan Leaf BEV and a comparable ICE car – the Ford Focus diesel.
| Nissan Leaf (electric) | Ford Focus (diesel) | |
|---|---|---|
| Purchase price | £24 000 (after £5000 subsidy) | £19 500 |
| Annual car tax | £0 | £125 |
| Annual fuel costs* | £230 | £1100 |
| Average annual parking and toll charges | £80 | £180 |
| Annual insurance | £280 | £550 |
| Annual interest on purchase loan | £600 | £490 |
Footnotes
* Assuming 10 000 miles/16 000 km a yearIf someone chose to buy a Leaf instead of a Focus, how long would it take for the lower running costs to repay the higher purchase cost?
Discussion
The Leaf costs £4500 more to buy than the Focus (even taking into account the £5000 purchase subsidy). The annual savings amount to £1255. Therefore the payback period is four years for these savings to repay that higher initial cost.
Without the (temporary) purchase subsidy, the Leaf would cost £9500 more than the Focus, pushing the payback period up to eight years. The chances are that the car would have been sold by then, so other financial factors – such as resale price and, possibly, battery replacement – would come into play.
This cost structure means that low-carbon vehicles can only have a relatively small price premium if they are to be financially competitive.
Despite the purchase subsidy that has cut the price of a BEV to around £24 000, there has been a slow uptake of these grants in the UK. Grants became available from January 2011, with £43m allocated until 31 March 2012 – enough to support the purchase of 8600 cars – yet in 2011, only a little over a thousand grants were made. The market launch of plug-in hybrids, considered next, may increase the rate of grant uptake; the scheme has now been widened to include electric vans and will run to 2015.
