1.1 The water cycle and drinking water
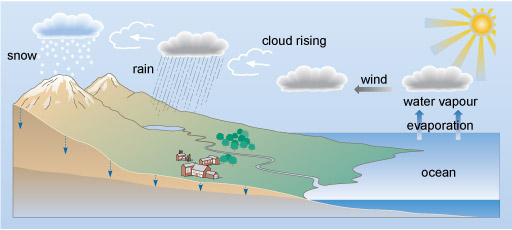
Water moves around the Earth in the water cycle (Figure 1). Natural water on Earth is not completely pure as compounds may dissolve at several points in the cycle and this may lead to pollution if above accepted guidelines.
Predict where inorganic pollutants might enter the water cycle?
At several steps in the water cycle inorganic compounds can become dissolved and, depending on their level, pollution may occur. For example:
- Anions such as nitrate and phosphate may become dissolved from agricultural fertilisers, sewage and the natural breakdown of organic matter.
- Air pollution from burning fuels can produce nitrogen oxides, NOx compounds and sulfur oxides, SOx compounds, to form dissolved nitrates and sulfates respectively.
- Cations such as calcium or magnesium may dissolve naturally due to the weathering of minerals in rocks or via anthropogenic, or human-derived, contamination of ground water by pollutants from industry, roads, or mining.
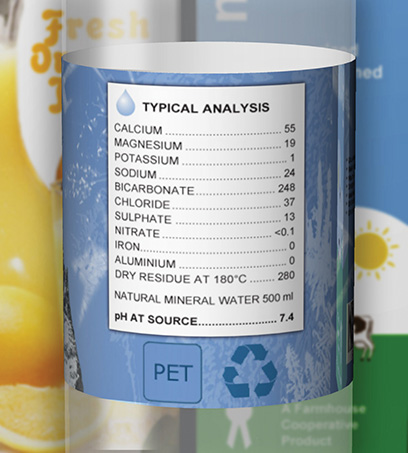
For drinking purposes water has to meet government guidelines but will not be absolutely pure as it is also a natural source of ions needed by the human body. Some typical values are shown in Figure 2 and Table 1.
What will the [H+(aq)] be in the water in Figure 1?
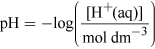 (Equation 1)
(Equation 1)Rearranging gives
[H+(aq)] = 10−pH mol dm−3 = 10−7.4 = 4.0 × 10−8 mol dm−3
| Ion | Concentration/mg l−1 | ||||
|---|---|---|---|---|---|
| Volvic® | Vittel® | Buxton® | Evian® | Tap water* | |
| calcium Ca2+ | 11.5 | 91 | 55 | 78 | 102 |
| magnesium Mg2+ | 8.0 | 19.9 | 19 | 24 | 8.81 |
| sodium Na+ | 11.6 | 7.3 | 24 | 5 | 49.1 |
| potassium K+ | 6.2 | - | 1 | 1 | n.a. |
| choride Cl− | 13.5 | - | 37 | 4.5 | 73.9 |
| nitrate NO3− | 6.3 | 0.6 | 3.5 | 20.6 | |
| sulfate SO42− | 8.1 | 105 | 13 | 10 | 120 |
| bicarbonate HCO3− | 71.0 | 258 | 248 | 357 | n.a. |
Footnotes
* value for the area containing The Open University in Milton Keynes; - = too small to measure; n.a.= not availableActivity 1 Ions in drinking water
Question 1
This activity aims to access up-to-date information about ions in drinking water. You will access the web pages of the Drinking Water Inspectorate, DWI (2015) of England and Wales [Tip: hold Ctrl and click a link to open it in a new tab. (Hide tip)] . (Hint: use the A-Z index.)
Search for information to answer the following questions:
Which ions in Table 1 are responsible for the hardness of water?
Answer
Cations such as calcium and magnesium.
Question 2
What is the World Health Organization (2015) guideline value for nitrate concentration in drinking water?
Answer
The World Health Organization (2015) guideline value for nitrate is 50 mg l−1 for drinking water and the EU also adopts this value.
Question 3
How does the concentration compare with the values for nitrate concentration in Table 1?
Answer
All the values for nitrate in Table 1 are well below 50 mg l−1.
Question 4
What is the allowed level of fluoride ions in drinking water? (Note this is often artificially added to water supplies.)
Answer
The maximum permitted value of fluoride in drinking water is 1.5 mg l−1.
You might wish to check the water quality report for your own water supplier. This is often available on the company website or see if the values for tap water in Milton Keynes in Table 1 have altered. Links to information on drinking water in other EU states can be found at European Commission (2015).