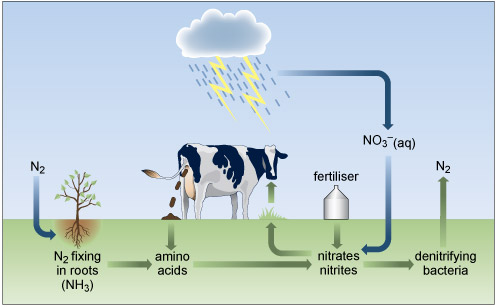
2.1 The nitrogen cycle
Cycling of nitrogen and its compounds through the environment involves a delicate balance of redox, atmospheric and biological processes (Figure 4) generally involving water as a solvent. Plants require nitrogen, which is absorbed in the form of nitrate or ammonium ions, for the synthesis of nitrogenous organic compounds, such as amino acids, which are incorporated into the tissues of the plant. However, some of the intrinsic nitrogen is lost upon removing a crop from the soil. This must be replaced to maintain the fertility of the soil.
Nitrogen has a versatile redox chemistry and displays several oxidation numbers in Figure 4.
What are the oxidation numbers of the nitrogen in ammonia (NH3), nitrogen gas, nitrite (NO2−) and nitrate (NO3−)?
The oxidisation number of hydrogen is generally +1 and oxygen is generally −2. The oxidation numbers of nitrogen in these compounds aree −3, 0, 3 and +5, respectively, to maintain the charge seen on the compounds. Note nitrogen compounds display oxidation numbers ranging from −3 to +5.
Like other elements in the second row of the Periodic Table, the nitrogen atom can form double bonds both to other nitrogen atoms and to atoms of some other elements of the second row, such as boron, carbon and oxygen.
Determine the resonance forms in the Lewis structure of nitrite.
There are two resonance forms (Structure 1).
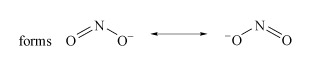 Structure 1
Structure 1