3.1.1 Phosphoric acid and fertilisers
Phosphoric acid is manufactured from phosphate minerals, and the pure acid forms low-melting crystals (Tm 42 °C). Commercial phosphoric acid is 85% phosphoric acid in water; this forms a syrup because the acid molecules are hydrogen-bonded to water molecules.
Most phosphoric acid is used to manufacture fertiliser. For example, so-called triple superphosphate fertilisers are manufactured from calcium phosphate-containing rock, such as Ca3(PO4)2, and phosphoric acid:
The farming of crops depletes soils of essential nutrients, such as phosphate and nitrate, which are replenished by applying fertilisers containing suitable inorganic compounds. Consequently, eutrophication from excess phosphate and nitrate in rivers and lakes remains an issue but now often occurs due to water run-off from agricultural land.
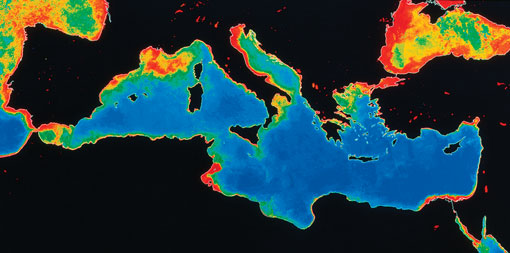
Figure 10 shows the excessive algal growth in the Mediterranean arising from excess nutrients in the water. These nutrients are regulated under the EU Water Framework Directive. For example, agricultural practices are altered to minimise pollution from applying nutrients to soils.
Phosphate can be removed from water by precipitation with lime, Ca(OH)2, forming hydroxyapatite (Equation 20), the same material which comprises bone and teeth:
Phosphate is recovered from wastewater during chemical sewage treatment, often as struvite, NH4MgPO4.6H2O, which can then be used as a fertiliser. Phosphate can also be recovered during biological water treatment where it is used in the growth of cell membranes, a process which ultimately forms biological solids or so-called sludge which can be used as a fertiliser.
