3.2 Oxoacids
Many p-block elements form oxoacids but here the focus will be on those of phosphorus with a few illustrative examples of other elements.The chemistry of phosphorous compounds in natural water and the body is not quite as simple as it has been treated so far, so the topic will be expanded upon here.
For instance, there are several oxoacids of phosphorus. The three most important are phosphoric acid, H3PO4, phosphorous acid, H3PO3, and hypophosphorous acid, H3PO2. Table 3 lists other names for these acids.
What is the oxidation number of phosphorus in H3PO4, H3PO3, and H3PO2?
The oxidation number is +5, +3 and +1, respectively.
Therefore H3PO4, H3PO3, and H3PO2 are also called phosphoric(V), phosphoric(III) and phosphoric(I) acid respectively.
| Formula and common name | Common anion name | Structure |
|---|---|---|
H3PO4 phosphoric acid or orthophosphoric acid | phosphate or orthophosphate | 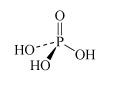 |
H3PO3 phosphorous acid or phosphonic acid | phosphite or phosphonate | 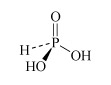 |
H3PO2 hydrophosphorous acid or phosphinic acid | hypophosphite or phosphinate | 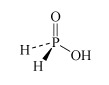 |
H4P2O7 diphosphoric acid | diphosphate | 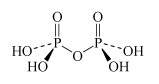 |
(HPO3)n (in the limit where n = ∞) metaphosphoric acid | metaphosphate | 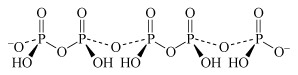 |
Some acids are polyprotic (or taking the alternative viewpoint polybasic) meaning they ionise stepwise, unlike hydrochloric acid, HCl, which is a monoprotic acid. Note only the protons attached to oxygen are ionisable. Phosphoric acid is triprotic undergoing three successive ionisations:
As phosphoric acid is triprotic, it can form three series of salts with a metal such as sodium: the dihydrogen phosphate, the hydrogen phosphate and the normal phosphate.
What would you expect to be the sodium salts of phosphorous acid, H3PO3?
Phosphorous acid is diprotic, forming the phosphite salts NaH2PO3 and Na2HPO3. Note one of the hydrogen atoms in H3PO3 is directly attached to the phosphorus and consequently does not undergo ionisation.
Note that Equations 21-23 have the form:
The pairs acid(1)/base(1) and acid(2)/base(2) are called a conjugate acid and base. Thus, H3O+ and H2O are a conjugate acid and base pair; so are H3PO4(aq) and H2PO4−(aq). An acid is transformed to its conjugate base by losing a proton and vice versa.
