4.1.1 Aluminium sulfate in water treatment
The Group 1 and Group 2 metals form carbonates, with those of Group 2 being insoluble in water. Aluminium carbonate, Al2(CO3)3, however, cannot be prepared; if aluminium sulfate is added to a solution containing carbonate or hydrogen carbonate ions, Al(OH)3 is precipitated and carbon dioxide is evolved:
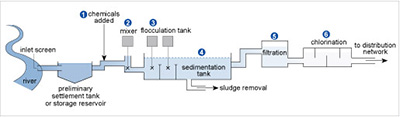
Figure 17 shows the stages in a typical water treatment process for river water. In stages 1 and 2 chemicals are added and mixed. Then in stage 3 flocculation or aggregation occurs involving large amounts of aluminium sulfate to clear the water of fine suspensions that are difficult to filter off, such as clay particles. The tiny particles usually carry surface negative charges, which repel each other and so remain in suspension.
The positively charged aluminium ions get between the negative particles, counteracting the repulsion and encouraging flocculation. Then, when Reaction 42 occurs because of the HCO3− ions usually present in natural waters, the particles sediment with the precipitate of aluminium hydroxide that forms in the sedimentation tank in stage 4. The water is filtered in stage 5 before disinfection in stage 6 via chlorination.
What role is Al3+(aq) playing in Equations 41 and 42?
Acids liberate CO2 from carbonate and hydrogen carbonate solutions, so here Al3+(aq) acts as an acid.
Then, Equation 43 becomes:
The positively charged alumnium ions get between the negative particles, counteracting the repulsion and encouraging flocculation. Then, when Reaction 42 occurs because of the HCO3 ions usually present in natural waters, the particles sediment with the precipitate of aluminium hydroxide that forms in the sedimentation tank in stage 4. The water is filtered in stage 5 before disinfection in stage 6 via chlorination.
Natural water generally requires disinfection before drinking to kill pathogenic or disease-causing microorganisms. Ultraviolet irradiation is one disinfection method and the major chemical disinfectants are ozone and chlorine. These compounds are thought to kill microorganisms by rupturing the cell membrane and reacting with proteins and enzymes within the cells. Once the chemical structures of proteins and enzymes have been altered they may either fall apart or adopt an unnatural state. Consequently, they fail to perform their roles and so the cell or bacteria dies.
Why can [Al(H2O)6]3+ be described as a Brønsted-Lowry acid in Equation 43?
Three of the six water molecules that were attached to the aluminium have been lost, but the other three have acted as proton donors, leaving aluminium associated with hydroxide ions instead of water molecules.
A more obvious sign of the acidic character of [Al(H2O)6]3+ is the fact that aqueous solutions of aluminium sulfate are acidic, unlike those of, say, sodium sulfate:
Again, a water molecule coordinated to Al3+ is transformed into an OH− ligand; at the same time, hydrated protons, H3O+, are generated.