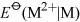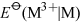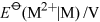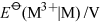4.1.5 The inert pair effect
Thallium is a Group 13 metal like aluminium, so why in its compounds is the +1 oxidation number prominent, such as in water the Tl+(aq) ion?
Consider first Group 2, magnesium is less readily oxidised than the metals beneath it. One sign of this is the less negative values of 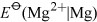 compared with those of the elements below it.
compared with those of the elements below it.
Table 5 compares values for Group 2 and Group 13 metals. Is the trend mentioned above maintained in Group 13?
No the trends are in opposite directions: thermodynamically speaking, aluminium is more readily oxidised to oxidation number +3 than gallium, indium or thallium.
|
| ||
|---|---|---|---|
| Mg | −2.36 | Al | −1.68 |
| Ca | −2.87 | Ga | −0.53 |
| Sr | −2.90 | In | −0.34 |
| Ba | −2.91 | Tl | +0.72 |
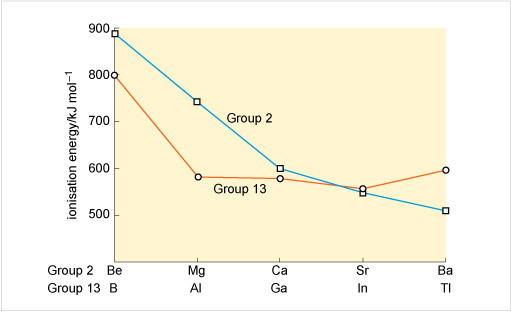
A possible reason for the reversal emerges from a comparison of the first ionisation energies of the Group 2 and Group 13 elements (Figure 20). Generally the ionisation energy:
- drops steeply when a new Period begins and
- increases overall across a Period as the nuclear charge builds up, as seen in Group 2.
Does Group 13 follow this general trend?
From boron to aluminium, there is the usual drop from the second row to the third row of the Group. Thereafter, the values remain unexpectedly high, most notably at thallium whose first ionisation energy exceeds that of aluminium.
Consider Figure 21. Why might the ionisation energies of gallium, indium and especially thallium be unexpectedly raised relative to those of aluminium?
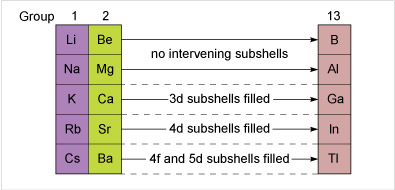
Aluminium follows immediately after a Group 2 element, but before gallium and indium, d shells must be filled. The ionisation energies are higher for gallium and indium because it takes longer to build up the nuclear charge. The effect is magnified at thallium, where prior filling of 5d and 4f subshells occurs.
Unexpectedly high ionisation energies for gallium, indium and thallium make conversion of the metals into ions more difficult. They are a major contribution to the greater resistance to oxidation revealed in Table 5. Such effects, however, do not obliterate the strong resemblance of gallium, indium and thallium to aluminium that their presence in the same Group implies. All three elements are metals, which react with fluorine or chlorine to form trihalides, all of which are solids at room temperature.
However the build-up of nuclear charge in the preceding 4f and 5d block elements leaves thallium's ionisation energies higher than expected. The higher oxidation number is therefore harder to attain, and the state most stable to oxidation or reduction is +1.
Determine the electronic configuration of Tl+.
[Xe] 4f14 5d10 6s2; the outer 6p electron has been lost, leaving two outer electrons in a full 6s shell.
The inert pair effect refers to the emergence at the bottom of Groups 13-15 of a stable lower oxidation number two fewer than the Group number. This is so called because the outer electronic configuration of the ion is a filled s2 subshell, which is presumed to be hard to remove during oxidation.
Watch Video 8 and determine which compounds aluminium commonly forms upon reaction with the halogens.
Download this video clip.Video player: Video 8Transcript: Video 8 The reaction of aluminium with some halogens.
End transcript: Video 8 The reaction of aluminium with some halogens.NARRATORAluminium forms solid trihalides when heated with the halogens. Here, we're heating aluminium in a stream of chlorine gas. An exothermic reaction produces white aluminium chloride. Adding aluminium foil to bromine produces aluminium tribromide in a spectacular reaction.The iodide has the same structure. Here, we mix aluminium with iodine. A drop of water dissolves a little of the iodine and allows the reactants to mix. The heat of the reaction is enough to sublime iodine, hence the purple vapour.Video 8 The reaction of aluminium with some halogens.Interactive feature not available in single page view (see it in standard view).Aluminium forms trihalides such as AlCl3.
The inert pair effect increases down the Group: AlCl, AlBr and AlI do not exist at room temperature, but the corresponding compounds of gallium and indium can be made by cooling a heated mixture of the metals and their trihalides:
They all, however, decompose in water, either evolving hydrogen or disproportionating to the metal and M3+(aq). Only in the case of thallium does a long-lived M+(aq) ion exist.
The inert pair effect is a relative phenomenon; consider whether it is also apparent if you study the two ionisation energies of the valence shell s electron pair.
For example, the atoms of the Group 2 elements have configurations of the type [FIS] ns2, (FIS refers to a filled inner shell) and in the +2 oxidation state, their ionic configuration is [FIS]. For a Group 2 element, the process
involves the loss of the outer s pair, and its enthalpy change is (I1 + I2), i.e. the sum of the first and second ionisation energies.
Rewrite this last sentence so that it applies to the loss of the outer s pair in Group 13.
For a Group 13 element, the process (Equation 51) involves the loss of the outer s pair, and its enthalpy change is (I2 + I3), the sum of the second and third ionisation energies.
M+(g) = M3+(g) + 2e−(g)(Equation 51)
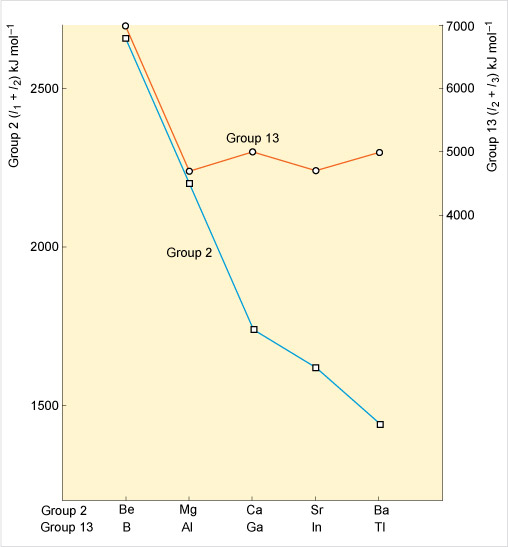
Figure 22 shows the change in these ionisation energy sums as one descends Groups 2 and 13. To make the comparison easier, the scales for each plot have been adjusted so that the slopes of the lines between Be and Mg, and between B and Al, are the same.
In Figure 22 do Groups 2 and 13 elements follow the trend you would expect?
Descending Group 2, the ionisation energy falls as expected because the outer shell becomes more remote from the nucleus: it gets progressively easier to remove the s pair.
Whilst in Group 13, the slope of the decrease changes abruptly at aluminium. Indeed, between Al and Ga, and In and Tl, there are increases. Unlike Group 2, it gets progressively harder to remove the s pair upon descending Group 13 due to an increasing nuclear charge from filling first the d subshell before Ga, In and Tl and also the f subshell before Tl. Consequently in Group 13, the +1 oxidation state becomes much more stable moving from Al to Tl.